Latent Heat and Freezing and Boiling Points
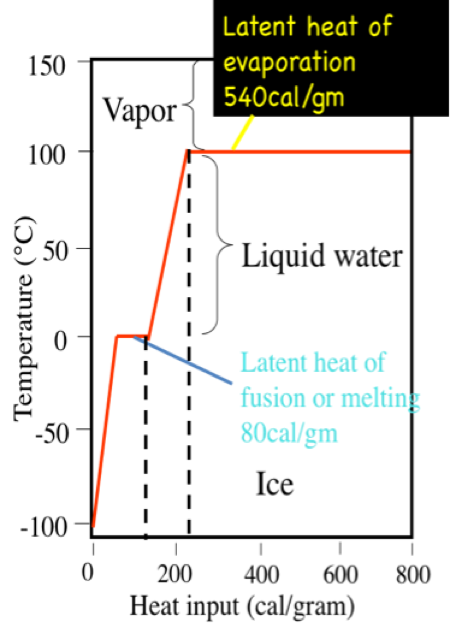
A calorie is the amount of heat it takes to raise the temperature of 1 gram (0.001 liters) of pure water 1 degree C at sea level. It takes 100 calories to heat 1 g. water from 0˚, the freezing point of water, to 100˚ C, the boiling point. However, 540 calories of energy are required to convert that 1 g of water at 100˚ C to 1 g of water vapor at 100˚ C. This is called the latent heat of vaporization. On the other hand, you would have to remove 80 calories from 1 g of pure water at the freezing point, 0˚ C, to convert it to 1 g of ice at 0˚ C. This is called the latent heat of fusion.
Interestingly, the latent heat and freezing and boiling points are controlled by the way water molecules interact with one another. Because molecules acquire more energy as they warm, the association of water molecules as clusters begins to break up as heat is added. In other words, the energy is absorbed by the fluid and molecules begin to dissociate from one another. Considerable energy is required to break up the water molecule clusters, thus there is relatively little temperature change of the fluid for a given amount of heating (this is the heat capacity measure), and, even at the boiling point, it takes far more energy to liberate water molecules as a vapor (parting them from one another). On the other hand, when energy is removed from water during cooling the molecules of water begin to coalesce into clusters and this process adds energy to the mix, thus offsetting the cooling somewhat.
Click here for a text description
