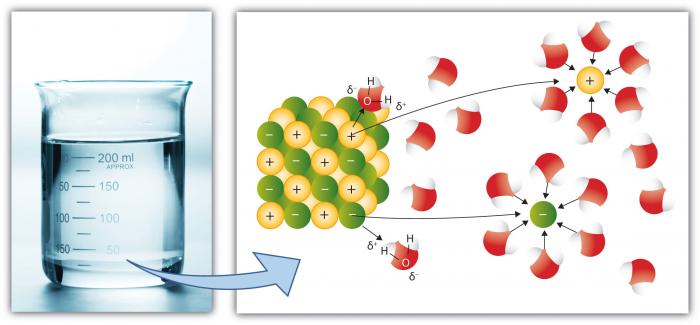
The Universal Solvent
This is, of course, another key property of water because more substances dissolve in water than any other common liquid. This is because the polar water molecule enhances "Dissolving Power." Dissolution involves breaking "salts" into component "ions." For example, NaCl (common salt) breaks down into the ions Na+ and Cl- because of the attraction for ions (atoms or groups of atoms with a charge) to water molecules is high.
Cations, such as Na (Sodium) have a net positive charge, whereas anions (such as Cl, Chloride) have a net negative charge. There are many individual elements and compounds that form ions. Thus, water can hold considerable concentrations of various chemical species depending on their particular properties. Note how the water molecules surround the individual ions, keeping them isolated from other ions in solution. This occurs until the capacity of water to isolate the ions is exceeded, at which point the solution is "saturated" with those ions and cannot dissolve more (salt will begin to precipitate—form a solid).