The Structure of Water: Properties
The Structure of Water: Properties
Studies have shown that clustering of water molecules occurs in solutions because of so-called hydrogen bonds (weak interaction), which are about 10% of the covalent water bond strength. This is not inconsiderable and energy is required to break the bonds, or is yielded by the formation of hydrogen bonds. Such bonds are not permanent and there is constant breaking and reforming of bonds, which are estimated to last a few trillionths of a second. Nonetheless, a high proportion of water molecules are bonded at any instant in a solution. But this structure leads to the other important properties of water.
We will consider, for the purposes of this course, only six of these important properties:
- Heat capacity
- Latent heat (of fusion and evaporation)
- Thermal expansion and density
- Surface Tension
- Freezing and Boiling Points
- Solvent properties
As mentioned above, these properties have importance to physical and biological processes on Earth. Effectively, large amounts of water buffer Earth surface environmental changes, meaning that changes in Earth-surface temperature, for example, are relatively minor. Thus, the high heat capacity of water promotes continuity of life on Earth because water cools/ warms slowly relative to land, aiding in heat retention and transport, minimizing extremes in temperature, and helping to maintain uniform body temperatures in organisms. However, there are other effects of water properties as well. Its low viscosity allows rapid flow to equalize pressure differences. Its high surface tension allows wind energy transmission to sea surface promoting downward mixing of oxygen in large water bodies such as the ocean. In addition, this high surface tension helps individual cells in organisms hold their shape and controls drop behavior (have you seen "An Ant's Life"?). Also, the high latent heat of evaporation is very important in heat/water transfer within the atmosphere and is a significant component of transfer of heat from low latitudes, where solar energy influx is more intense to high latitudes that experience solar energy deficits.
Video: Water - Liquid Awesome: Crash Course Biology #2 (11:16)
Take a few minutes to learn why water is the most fascinating and important substance in the universe.
Ah, hello there, here at crash course HQ we like to start out each day with a nice healthy dose of water in all its three forms it is the only substance on all of our planet Earth that occurs naturally in solid liquid and gas forms and to celebrate this magical bond between two hydrogen atoms and one oxygen atom here today we are going to be celebrating the wonderful life-sustaining properties of water but we're going to do it slightly more clothed. Much better.
We left off here at the biology crash course we're talking about life and the rather important fact that all life as we know it is dependent upon there being water around I'm just an astronomers are always looking out into the universe trying to figure out whether there is life elsewhere because you know that is kind of the most important question that we have right now I was getting really excited when they find water someplace particularly liquid water, and this is one reason why I and so many other people geeked out so hard last December when on Mars a seven-year-old rover Opportunity found a 20 inch long vein of gypsum that was almost certainly deposited by like long-term liquid water on the surface of Mars and this was probably billions of years ago. And so it's going to be hard to tell whether or not the water that was there resulted in some life, but maybe we can figure that out and that would be really exciting. But why do we think that water is necessary for life? Why does water on other planets get us so friggin excited?
So let's start out by investigating some of the amazing properties of water. In order to do that we're gonna have to start out with this the world's most popular molecule or at least the world's most memorized molecule, we all know about it good old h2o. Two hydrogens one oxygen the hydrogen's each sharing an electron with oxygen in what we call a covalent bond. So as you can see you have drawn my water molecule in a particular way and this is actually the way that it appears it is v-shaped because this big ol oxygen atom is a little bit more greedy for electrons. It has a slight negative charge whereas this area here with the hydrogen atoms has a slight positive charge thanks to this polarity all water molecules are attracted to one another so much so that they actually stick together and these are called hydrogen bonds and we talked about the last time essentially what happens is that the positive pole around those hydrogen atoms bonds to the negative pole around the oxygen atoms of a different water molecule and so it's a weak bond but look they're bonding seriously I cannot overstate the importance of this hydrogen bond so when your teacher asks you what's important about water start out with hydrogen bonds and you should put it in all gaps and maybe some sparkles around it one of the cool properties that results from these hydrogen bonds is a high cohesion for water which results in high surface tension cohesion is the attraction between - like things like attraction between one molecule of water and another molecule of water water has the highest cohesion of any nonmetallic liquid and you can see this if you put some water on some wax paper or some Teflon or something where the water beads up like that some some leaves of plants do it really well. It's quite cool since water adheres weakly to the wax paper or to the plant but strongly to itself the water molecules are holding those droplets together in a configuration that creates the least amount of surface area it's this high surface tension that allows some bugs and even I think one lizard and also one Jesus to be able to walk on what a Cui's of force of water does its limits.
Of course, there are other substances that water quite likes to stick to. Take glass for example, this is called adhesion and the water is spreading out here instead of beating up because the adhesive forces between the water and the glass are stronger than the cohesive forces of the individual water molecules in the bead of water adhesion is attraction between two different substances so in this case the water molecules and glass molecules these properties lead to one of my favorite things about water is the fact that it can defy gravity. That really cool thing that just happened is called capillary action and explaining it can be easily done with what we now know about cohesion and adhesion thanks to adhesion the water molecules are attracted to the molecules in the straw but as the water molecules adhere to the straw other molecules are drawn in by cohesion following those fellow water molecules thank you cohesion the surface tension created here causes the water to climb up the straw and it will continue to climb until eventually gravity pulling down on the weight of the water and the straw overpowers the surface tension. The fact that water is a polar molecule also makes it really good at dissolving things.
It's a good solvent, scratch that water isn't a good solvent, it's an amazing solvent! There are more substances that can be dissolved in water than in any other liquid on earth and yes that includes the strongest acid that we have ever created these substances that dissolve in water is sugar or salt being ones that we're familiar with are called hydrophilic and they are hydrophilic because they are polar and their polarity is stronger than the cohesive forces of the wall, so when you get one of these polar substances in water it's strong enough that it breaks all the little cohesive forces. All those little hydrogen bonds and instead of hydrogen bonding to each other the water will hydrogen bond around these polar substances table salt is ionic and right now it's being separated into ions as the poles of our water molecules interact with it but what happens when there is a molecule that cannot break the cohesive forces of water it can't penetrate and come into it basically what happens when that substance can't overcome the strong cohesive forces of water and can't get inside of the water? That's what we get what we call hydrophobic substance or if something that is fearful of water.
These molecules lacked charged poles they are nonpolar and are not dissolving in water because essentially they're being pushed out of the water by water's cohesive forces water we may call it the universal solvent but that does not mean that it dissolves everything there have been a lot of eccentric scientists throughout history but all this talk about water got me thinking about perhaps the most eccentric of the eccentrics a man named Henry Cavendish he communicated with his female servants only via notes and added a staircase to the back of his house to avoid contact with his housekeeper. Some believe he may have suffered from a form of autism but just about everyone will admit that he was a scientific genius. He's best remembered as the first person to recognize hydrogen gas as a distinct substance and to determine the composition of water in the 1700s most people thought that water itself was an element but Cavendish observed that hydrogen which he called inflammable air reacted with oxygen known then by the awesome name defroster gated air to form water. Cavendish didn't totally understand what he'd discovered here in part because he didn't believe in chemical compounds he explained his experiments with hydrogen in terms of a fire like element called phlogiston nevertheless his experiments were groundbreaking like his work and determining the specific gravity basically the comparative density of hydrogen and other gases with reference to common air it's especially impressive when you consider the crude instruments he was working with this for example is what he made his hydrogen gas with he went on not only to establish an accurate composition of the atmosphere but also discovered the density of the earth not bad for a guy who was so painfully shy that the only existing portrait of him was sketched without his knowledge so for all of his decades of experiments only published about 20 papers in the years after his death researchers figured out that Cavendish had actually pre discovered Richter's law Ohm's law Coulomb's law several other laws that's a lot of freakin laws and if he had gotten credit for them all we would have had to deal with like Cavendish's eight flaw and Cavendish's fourth law. So I for one am glad that he didn't actually get credit.
We're gonna do some pretty amazing science right now you guys are not going to believe this okay you ready, it floats. Yeah I know you're not surprised by this but you should be because everything else when it's solid is much more dense than when it's liquid just like gases are much less dense than liquids are but that simple characteristic of water that it's solid form floats is one of the reasons why life on this planet as we know it is possible and why is it that solid water is less dense than liquid water while everything else is the exact opposite of that. Well you can thank your hydrogen bonds once again so at around 32 degrees Fahrenheit or zero degrees Celsius if you're a scientist or from a part of the world where things make sense water molecules start to solidify and the hydrogen bonds in those water molecules form crystalline structures that space molecules apart more evenly in turn making frozen water less dense than its liquid form so in almost every circumstance of floating water ice is a really good thing if I swear denser than water it would freeze and then sink and then freezing than sinking than freezing than sink so just trust me on this one you don't want to live on a world where I sinks not only would it totally wreak havoc on aquatic ecosystems which are basically how life formed on the earth in the first place it would also you know all the ice and the North Pole would sink and then all of the water everywhere else would rise and we wouldn't have any land that would be annoying.
There's one more amazing property of water that I'm forgetting so why is it so hot in here. Oh heat capacity yes water has a very high heat capacity and probably that means nothing to you but basically it means that water is really good at holding on to heat which is why we like to put hot water bottles in our bed and with them when we're lonely but aside from artificially warming your bed it's also very important that it's hard to heat up and cool down the oceans significantly they become giant heat sinks that regulate the temperature and the climate of our planet which is why for example it's so much nicer in Los Angeles where the ocean is constantly keeping the temperatures the same then it is and say Nebraska on a smaller scale we can see waters high heat capacity really easily and visually by putting a pot with no water in it on a stove and seeing how badly that goes but then you put a little bit of water in it and it takes forever to frickin boil oh and if you haven't already noticed this or when water evaporates from your skin it cools you down now that's the principle behind sweating which is an extremely effective though somewhat embarrassing part of life but this is an example of another incredibly cool thing about water when my body gets hot and it sweats that heat excites some of the water molecules on my skin to the point where they break those hydrogen bonds and they evaporate away and when they escape they take that heat energy with them leaving me cooler lovely well this wasn't exercise though I don't know why sweating so much it could be the spray bottle that I keep spraying myself with her maybe it's just because this is such a high-stress enterprise trying to teach you people things I think I need some water but while I'm drinking ah there's review for all of the things that we talked about today if you there are a couple things that you're not quite sure about just go back and watch them it's not going to take a lot of your time and you're going to be smarter. I promise you're going to do so well on that test you either don't or do have coming up okay bye
Heat Capacity
Heat Capacity
Water does not give up or take up heat very easily. Therefore, it is said to have a high heat capacity. In Colorado, it is common to have a difference of 20˚ C between day and night temperatures. At the same time, the temperature of a lake would hardly change at all. This property originates because energy is absorbed by water as molecules are broken apart or is released by molecules of water associating as clusters.
Video: Heat Capacity of Water (01:13)
Take a few minutes to watch the video below to help you understand heat capacity.
The video begins by showing two candles and two balloons. One balloon (the yellow one) is partially filled with water. Both candles are lit and the ballons are moved so they are directly on top of the flame. The balloon without water bursts. This happens because the water absorbs the heat from the flame. The balloon is then picked up to reveal that the bottom of the balloon is burnt.
Latent Heat and Freezing and Boiling Points
Latent Heat and Freezing and Boiling Points
A calorie is the amount of heat it takes to raise the temperature of 1 gram (0.001 liters) of pure water 1 degree C at sea level. It takes 100 calories to heat 1 g. water from 0˚, the freezing point of water, to 100˚ C, the boiling point. However, 540 calories of energy are required to convert that 1 g of water at 100˚ C to 1 g of water vapor at 100˚ C. This is called the latent heat of vaporization. On the other hand, you would have to remove 80 calories from 1 g of pure water at the freezing point, 0˚ C, to convert it to 1 g of ice at 0˚ C. This is called the latent heat of fusion.
Interestingly, the latent heat and freezing and boiling points are controlled by the way water molecules interact with one another. Because molecules acquire more energy as they warm, the association of water molecules as clusters begins to break up as heat is added. In other words, the energy is absorbed by the fluid and molecules begin to dissociate from one another. Considerable energy is required to break up the water molecule clusters, thus there is relatively little temperature change of the fluid for a given amount of heating (this is the heat capacity measure), and, even at the boiling point, it takes far more energy to liberate water molecules as a vapor (parting them from one another). On the other hand, when energy is removed from water during cooling the molecules of water begin to coalesce into clusters and this process adds energy to the mix, thus offsetting the cooling somewhat.
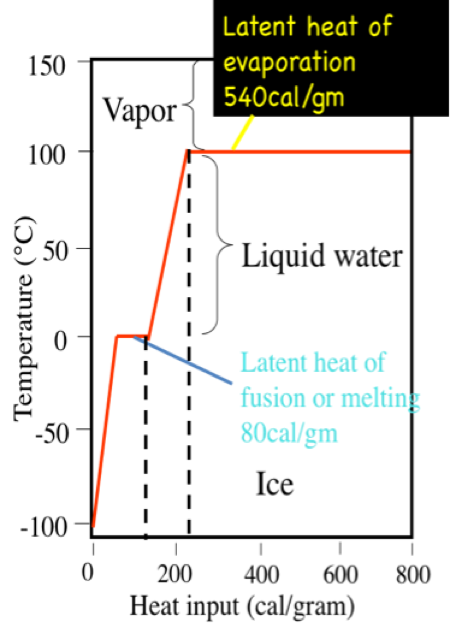
Click here for a text description
Thermal Expansion and Density
Thermal Expansion and Density
When water is a liquid, the water molecules are packed relatively close together but can slide past each other and move around freely (as stated earlier, that makes it a liquid). Pure water has a density of 1.000 g/cm3 at 4˚ C. As the temperature increases or decreases from 4˚ C, the density of water decreases. In fact, if you measure the temperature of the deep water in large, temperate-latitude (e.g., the latitude of PA and NY) lakes that freeze over in the winter (such as the Great Lakes), you will find that the temperature is 4˚ C; that is because fresh water is at its maximum density at that temperature, and as surface waters cool off in the Fall and early Winter, the lakes overturn and fill up with 4˚ C water.
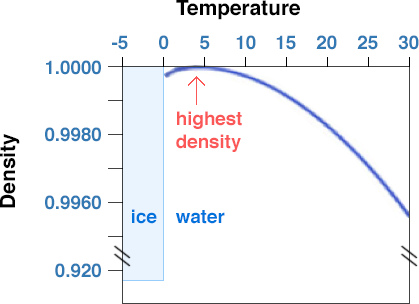
However, as dissolved solids are added to pure water to increase the salinity, the density increases. The density of average seawater with a salinity of 35 o/oo (35 g/kg) and at 4˚ C is 1.028 g/cm3 as compared to 1.000g/cm3 for pure water. As you add salts to seawater, you also change some other properties. Incidentally, increasing salinity increases the boiling point and decreases the freezing point. Normal seawater freezes at -2˚ C, 2˚ C colder than pure water. Increasing salinity also lowers the temperature of maximum density. This effect also helps explain why you are supposed to add salt to ice when making ice cream or to add salt to water when cooking spaghetti (although, in this case, the effect on boiling point is minor and the added salt is mainly for flavor).
When water freezes, however, bonds are formed that lock the molecules in place in a regular (hexagonal) pattern. For nearly every known chemical compound, the molecules are held closer together (bonded) in the solid state (e.g., in mineral form or ice) than in the liquid state. Water, however, is unique in that it bonds in such a way that the molecules are held farther apart in the solid form (ice) than in the liquid. Water expands when it freezes making it less dense than the water from which it freezes. In fact, its volume is a little over 9% greater (or density ca. 9% lower) than in the liquid state. For this reason, ice floats on the water (like an ice cube in a glass of water). This latter property is very important for organisms in the oceans and/or freshwater lakes. For example, fish in a pond survive the winter because ice forms on top of a pond (it floats) and effectively insulates (does not conduct heat from the pond to the atmosphere as efficiently) the rest of the pond below, preventing it from freezing from top to bottom (or bottom to top).
If water did not expand when freezing, then it would be denser than liquid water when it froze; therefore it would sink and fill lakes or the ocean from bottom to top. Once the oceans filled with ice, life there would not be possible. We are all aware that expansion of liquid water to ice exerts a tremendous force. Have you or a family member (you wouldn't admit to this would you?) ever left a full container of water with a tight-fitting lid (or even a can of soda?) in the freezer? In other words, 10 cups of water put into the freezer is going to turn into 11 cups of ice when it freezes (oops). The force of crystallization of ice is capable of bursting water pipes and causes expansions of cracks in rocks, thus accelerating the erosion of mountains!
A rough sketch of water molecules in ice crystal form is below.
Surface Tension
Surface Tension
Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly attracted to the molecules of water below them by their hydrogen bonds. If the diameter of the container is decreased to a very fine bore, the combination of cohesion, which holds the water molecules together, and the adhesive attraction between the water molecules and the glass container will pull the column of water to great heights. This phenomenon is known as capillarity. This is a key property that allows trees to stand high, for example, because surface tension stiffens stems and trunks. Plants "wilt" because they are unable to acquire sufficient water to maintain the required surface tension. And, of course, water droplets (rain) and fog condensing as droplets on surfaces are a function of water's surface tension. Without this property, water would be a slimy coating and cells would not have shape. Surface tension decreases with temperature and salinity.
Video: Amusing Surface Tension Experiment (02:39)
Please take a few minutes to watch this amusing video to learn more about the surface tension of water.
Inside your clicky pen is a science experiment waiting to spring forth. Fill a cup with water. Place the spring from your clicky pen ever so gently into the water it floats. Why? Because the middle of the spring is lighter than water? No Diana, you buffoon, metal is not lighter than water and as much as this spring resembles the Titanic one of them is doing a better job of staying afloat. But wait now I will activate the evil goo of death okay? Now before I bring travesty and devastation to this display of tensile forces I will explain it because it's cool enough to destroy the water holds up the spring because the h2o molecules on the surface of the water are bonded together quite tight. These surface molecules have fewer neighbors than the rest of the molecules and the one could say they're exposed like parts of Janet Jackson I never wanted to see. Therefore they use all their bonding power to hold on tight to their neighbors below and on all sides so consequently. They're pulled down which creates a pressure on the surface, pulling it toward the rest of the water in the cup stay with me don't leave my page yet this pressure creates a cushion or net that the spring can rest on comfortably and now for the destruction of it all and don't even attempt to stop me because in my hands is soap the soap that will break the hydrogen bonds and the molecules on the surface of the water because my silk molecules will attach to the h2o and steal them away from their girlfriends and childrens and wives. I just said childrens. Well, there you have it.
The Universal Solvent
The Universal Solvent
This is, of course, another key property of water because more substances dissolve in water than any other common liquid. This is because the polar water molecule enhances "Dissolving Power." Dissolution involves breaking "salts" into component "ions." For example, NaCl (common salt) breaks down into the ions Na+ and Cl- because of the attraction for ions (atoms or groups of atoms with a charge) to water molecules is high.
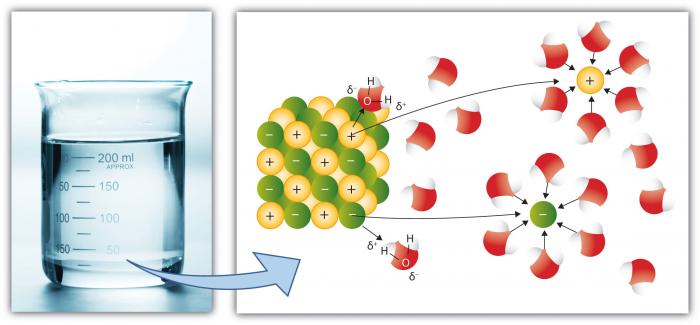
Cations, such as Na (Sodium) have a net positive charge, whereas anions (such as Cl, Chloride) have a net negative charge. There are many individual elements and compounds that form ions. Thus, water can hold considerable concentrations of various chemical species depending on their particular properties. Note how the water molecules surround the individual ions, keeping them isolated from other ions in solution. This occurs until the capacity of water to isolate the ions is exceeded, at which point the solution is "saturated" with those ions and cannot dissolve more (salt will begin to precipitate—form a solid).