What's a Mineral?
A mineral is a homogeneous, naturally occurring, solid inorganic substance with a definable chemical composition and an internal structure characterized by an orderly arrangement of atoms, ions, or molecules in a lattice.
Wow, that's kind of a mouthful, but each part of the definition is easy to explain.
- Homogeneous: The material is the same through and through. Are any of you old enough to remember when milk came in bottles and you had to skim the cream off the top? Now, the all milk available in grocery stores is homogenized, which means it has been processed so that the cream doesn't separate.
- Naturally occurring: True minerals are not formed by the activities of people. For example, manufactured industrial diamonds are “synthetic minerals.”
- Solid: Gases and liquids are not minerals.
- Inorganic substance: Oil, gas, fat, and plastic are organic and therefore not minerals.
- Definable chemical composition: Minerals have a chemical formula you can write down.
- Orderly arrangement of atoms in a lattice: The atom, ions, or molecules occur in a specific repeating pattern called a crystal lattice.
Quiz yourself!
- Is a snowflake a mineral? (check your answer)
- Is glass a mineral? (check your answer)
Did you know?
It is a common urban myth that glass acts like a liquid over long timescales. This myth, mostly perpetuated by tour guides in cities with old windows, is "proven" by pointing out to onlookers that the window glass in old buildings is thicker at the bottom. This is used as "evidence" that the glass flows downward over time due to gravity and has piled up at the bottom of the pane. Actually, the fact that old windows are thicker at the bottom is an artifact of an old method of glass-blowing, called the "crown glass procedure." In this method, still-molten glass was spun on a disc to flatten it as it cooled. This had the effect of making the disc fatter at the edges. Then the panes of glass were cut with an asymmetrical thickness built in to them. These panes were then usually installed with the thick end down because that is more stable. For a more detailed analysis of this topic, check out Plumb, 1989. "Antique windowpanes and the flow of supercooled liquids." J. Chem. Educ., 66, pp. 994-996.
Isotopes and Polymorphs
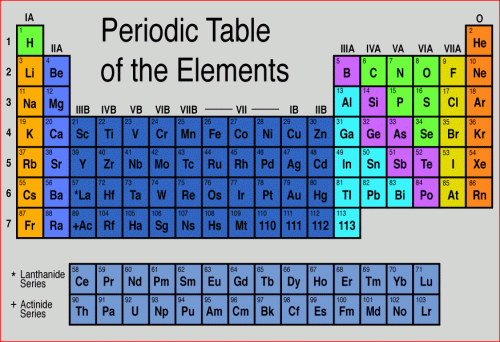
In order to discuss the the classification of various types of minerals, let's take a quick look at the Periodic Table of the Elements and review some simple atomic chemistry. All elements in the periodic table are made of one kind of atom, with a specific number of protons in its nucleus. The number of protons in the nucleus is the atomic number of the element. Below is an image of the periodic table. I assume this is familiar to you from high school and college!
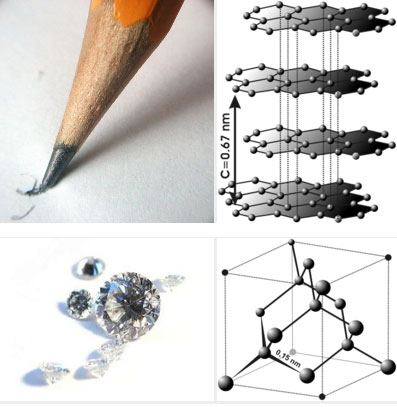
Let's take the element carbon, for example. Its atomic number is 6. That means it has 6 protons in its nucleus and 6 electrons orbiting. It can have different numbers of neutrons in its nucleus (6,7,8 are common). Two atoms with the same number of protons, but different numbers of neutrons are isotopes. Common isotopes of carbon are carbon-12 and carbon-14, denoted like this: C12 and C14. The superscripts in the examples for carbon are the atomic weights of the isotopes. C12 has 6 protons and 6 neutrons in its nucleus, so its atomic weight is 12. C14 has 6 protons and 8 neutrons in its nucleus, so its atomic weight is 14. Two minerals that have the same chemical composition but different crystal lattice structures are called polymorphs. For example, graphite and diamond are both pure carbon, but the way the carbon atoms are arranged is completely different, giving rise to their very different chemical and physical properties (see images below). In graphite, the carbon atoms are arranged in sheets that are weakly bonded to each other. In a diamond, the lattice structure involves a much stronger bond framework.
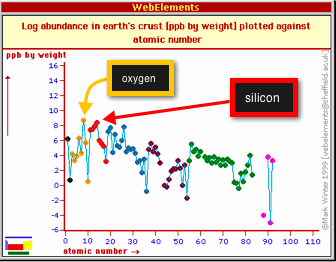
Silicate minerals
Not all of the elements in the Periodic Table are particularly common in the Earth's crust. Most minerals are formed from different arrangements of just a handful of the most commonly occurring elements. By weight, the two most abundant elements in the crust are oxygen and silicon. The figure below shows the abundance of elements in the crust as parts-per-billion by weight plotted vs. the atomic number of the element. See that oxygen (atomic number = 8, shown by one of the yellow dots) and silicon (atomic number = 14, shown by one of the red dots) are the highest.