5.2a Starch
We briefly addressed what starch is in Lesson 5. Now, we’ll go into a little more depth. In plants, starch has two components: amylose and amylopectin. Amylose is a straight-chain sugar polymer. Normal corn has 25% amylose, high amylose corn has 50-70% amylose and waxy corn (maize) have less than 2%. The rest of the starch is composed of amylopectin. Its structure is branched and is most commonly the major part of starch. Animals contain something similar to amylopectin, called glycogen. The glycogen resides in the liver and muscles as granules.
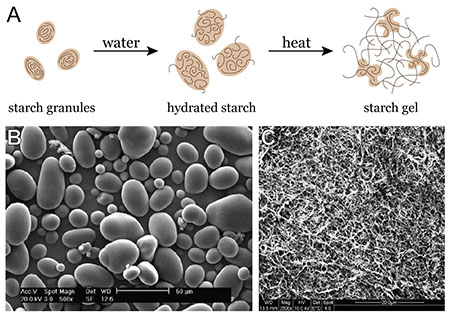
You can visit howstuffworks.com to see a schematic of what amylopectin looks like in a granule (see 'How Play-Doh Works') and then strands of the compound. The figure below shows some micrographs of starch as it begins to interact with water. When cooking with starch, you can make a gel from the polysaccharide. (A) This part of the figure shows polysaccharides (lines) packed into larger structures called starch granules; upon adding water, the starch granules swell and polysaccharides begin to diffuse out of the granules. Heating these hydrated starch granules helps polysaccharide molecules diffuse out of the granules and form a tangled network. (B) This is an electron micrograph of intact potato starch granules. (C) This is an electron micrograph of a cooked flaxseed gum network.
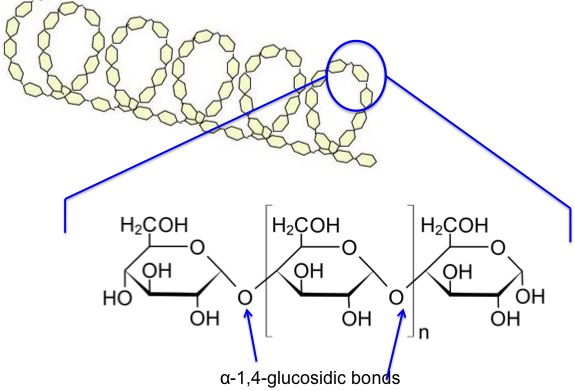
Now, let’s look at the starch components on a chemical structure basis. Amylose is a linear molecule with the α-1,4-glucosidic bond linkage. Upon viewing the molecule on a little larger scale, one can see it is helical. It becomes a colloidal dispersion in hot water. The average molecular weight of the molecule is 10,000-50,000 amu, and it averages 60-300 glucose units per molecule. Figure 6.5 depicts the chemical structure of amylose.
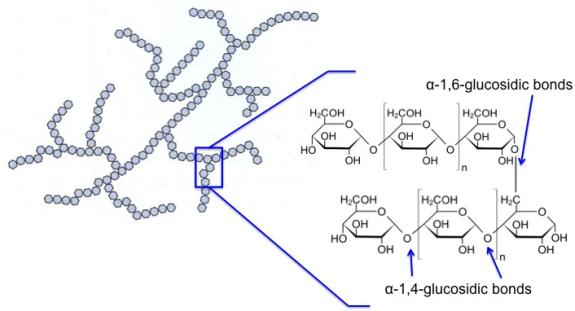
Amylopectin is branched, not linear, and is shown in the figure below. It has α-1,4-glycosidic bonds and α-1,6-glycosidic bonds. The α-1,6-glycosidic branches occur for about 24-30 glucose units. It is insoluble compared to amylose. The average molecular weight is 300,000 amu, and it averages 1800 glucose units per molecule. Amylopectin is about 10 times the size of amylose.