11.3 Microbial Fuel Cells
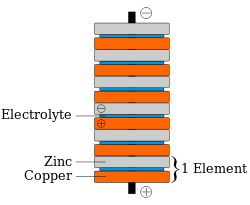
A microbial fuel cell is a bio-electrochemical device that can convert chemical energy directly into electrical energy. But first, let’s go over what a fuel cell is. A fuel cell is a battery of sorts. So, what is a battery? A battery is when two different types of metals are connected together through what is called an electrolyte. One metal is an anode, which is a metal that “wants” to give off electrons when under the right conditions. One metal is a cathode, which is a metal that “wants” to accept electrons when under the right conditions. When these two metals are in close proximity, and there is a fluid that will conduct the electrons (an electrolyte), then the flow of electrons from one metal to the other can occur. And we can capture that flow to extract electricity. Batteries that we use in remotes for televisions will eventually get used up and need to be replaced. This is an example of a primary battery. The figures below show a generic cell, a stack of cells using zinc and copper, and a picture of a Voltaic cell as created by Alessandro Volta (inventor).
We can also have secondary batteries, where the flow of electricity is established to provide electrical energy, but we can also apply electricity to the battery to reverse the flow of electrons and regenerate the life of the battery. While regenerated batteries don’t last forever, you can definitely get your money’s worth because you can regenerate them.
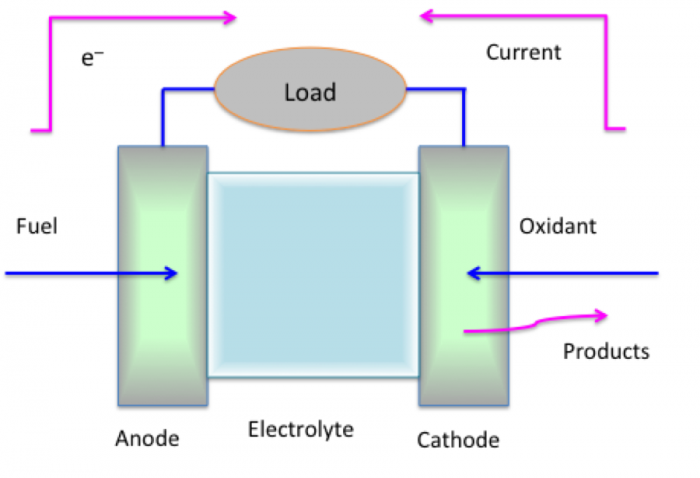
Fuel cells are also a sort of battery, but the materials are different and flow continuously to produce electricity. The figure below shows a generic fuel cell. As with a battery, it has an anode, cathode, and electrolyte. The anode typically uses hydrogen as the fuel, on the left side of the figure, and oxygen as the oxidant used in the cathode, on the right side of the figure. The electrolyte contains a fluid but also a membrane that removes the protons from the hydrogen, leaving the electrons to flow, and allowing oxygen to accept the protons to form water. Typically, these cells are run on hydrogen and oxygen, but we get electrical energy out rather than heat from burning hydrogen and oxygen.
At the anode, hydrogen reacts as shown in reaction 18:
(18) H2 → 2H+ + 2e-
This is an oxidation reaction that produces protons and electrons at the anode. The protons then migrate through an acidic electrolyte, and the electrons travel through an external circuit. Both arrive at the cathode to react with the oxidant, oxygen, as shown in reaction 19:
(19) ½ O2 + 2H+ + 2e- → H2O
This is the reduction reaction, where oxygen can be supplied purely or in the air. Essentially, the total circuit is completed through the mass transfer of protons in the electrolyte and external electrical circuit. There will be a small amount of heat lost through the electrodes. The overall reaction is shown in reaction 20:
(20) H2 + ½ O2 → H2O + work + waste heat
The water and waste heat are the by-products and must be removed on a continuous basis. An ideal voltage from this reaction is 1.22 volts, but less than that voltage will be realized. Other issues include the use of fuel different from hydrogen (methanol, hydrocarbons, etc.) and the fact that fuel cells produce direct current (DC) when most applications require alternating current. This little bit of background has been provided so a short discussion on microbial fuel cells can be had.
We are not going to go into great depth on microbial fuel cells, as some of the biochemistry can be complex. I will provide you with some generic information on how a microbial fuel cell is set up. These are bio-electro-chemical devices, which convert chemical energy directly into electrical energy. As we have discussed before, there are some steps that need to occur. Cellulose is hydrolyzed into sugars, i.e., glucose. The sugars are fermented into short-chain fatty acids, alcohols, hydrogen, and carbon dioxide. Finally, electricigenesis takes place, producing electricity, and carbon dioxide is carried through. Electricigenesis converts chemical energy to electrical energy by the catalytic reaction of microorganisms. The anode is anaerobic, and the anode chamber contains microbes and feedstock. The fuel is oxidized by microorganisms, which generate CO2, electrons, and protons. The cathode is the aerobic chamber, and just like other fuel cells, a proton exchange membrane separates the two chambers and allows only protons (H+ ions) to pass.
There are two types of microbial fuel cells (MFCs): mediator or mediator-less. The mediator type was demonstrated in the early 20th century and uses a mediator: a chemical that transfers electrons from the bacteria in the cell to the anode. Some of these chemicals include thionine, methyl viologen, methyl blue, humic acid, and neutral red. These chemicals are expensive and toxic. Mediator-less MFCs are a more recent development, from the 1970s. These types of cells have electrochemically active redox proteins such as cytochromes on their outer membrane that can transfer electrons directly to the anode. Some electrochemically active bacteria are Shewanella putrefaciens and Aeromomas hydrophila. Some bacteria have pili on the external membrane, which allows for electron production through the pili. They are beginning to find commercial use in the treatment of wastewater. I’ve included some YouTube videos explaining how the MFCs work. The first video is a brief but complete explanation of how an MFC works (2:03).
[MUSIC PLAYING]
PRESENTER: The MudWatt Microbial Fuel Cell is a bio-electrical device that uses the natural metabolisms of microbes found within soil to produce electrical energy. Here's how it works.
The MudWatt is comprised of two graphite felt electrodes-- the anode and the cathode-- held within a durable airtight container. The piece of electronics on top is used for experimentation and also features an LED light which blinks using the power of the microbes within your soil. The user simply fills the MudWatt with wet soil, burying the anode while resting the cathode on top.
In this configuration, a healthy community of electricity-generating microbes will develop on the surface of the anode in a matter of days. These bacteria have unique metabolic abilities which enable them to respire the sugars and nutrients within the soil and deposit electrons onto the anode as part of their natural metabolism. Protons and carbon dioxide are released into the soil as metabolic byproducts and diffuse toward the cathode.
Once transferred to the anode, the electron then travels through the electrode wire, through the MudWatt electronics to the cathode. While passing through the electronics, this electrical current will light the LED light on top, giving you a visual indication that your microbes are healthy and happy.
At the cathode, the electron interacts with oxygen in the air, as well as protons coming from the anode, to form water. The carbon dioxide originating from the anode is released into the air. The cycle continues, limited only by the availability of nutrients within the soil and oxygen within the air.
[MUSIC PLAYING]
The next video is a little longer and goes into a little more depth of what was described above (7:23).
[MUSIC PLAYING]
PRESENTER: Inspiration to build bio-electrochemical systems came from a discovery of certain microbes that live in soil. These bacteria swim up to the solid metal-- such as iron-- transfer electrons to the metal, dissolving it in process. This is similar to aerobic bacteria that transfer electrons to molecules of oxygen during respiration. The electron transfer generates electricity. To where there's electricity, there is power.
PRESENTER: To harvest the electricity, a bio-electrochemical fuel cell is used. This system consists of two compartments-- an anode compartment and a cathode compartment. These two compartments are separated by a membrane. A biofilm grows on the end of it.
[MUSIC PLAYING]
An organic feed stream, such as wastewater, enters the fuel cell, where it is oxidized by the biofilm. Simultaneously, oxidized products leave the fuel cell. The oxidation of organics-- for instance, acetate-- produces electrons and protons. This half-reaction releases a certain amount of energy.
Electrons are conducted over the wire while protons move through the membrane to the cathode to uphold electron neutrality. Oxygen is supplied to the cathode chamber. There accepts the electrons and reacts with the protons to form water. This half-reaction also releases a certain amount of energy.
[MUSIC PLAYING]
The theoretical maximum energy gain is determined by combining both half-reactions. However, resistances are found in multiple layers of the fuel cell.
[MUSIC PLAYING]
The Olmec losses are found in the electrical wire and in the proton transfer from the anode to the cathode. Concentration losses occur when the rate of mass transferred either to anode or cathode compartment limits the rate of product formation. Bacterial metabolic losses can be described by the amount of energy that is used by the microbes to grow.
The energy is harvested to form a protein gradient over the inner membrane. Activation losses are described by the capacity of the biofilm to transfer the electrons to the anode. Certain organisms can grow conductive nanowires-- called pili-- that directly interact with anode to transfer the electrons.
[MUSIC PLAYING]
[BIRDS CHIRPING]
PRESENTER: OK. Today, we're out in the wild. And we're looking for some sludge to power our bio-battery. I think this a nice spot. Ah, it's perfect.
PRESENTER: Another application of bio-electrochemical systems is the production of chemicals. In this case power must be applied to biofilm by external source. Electrons are produced by the oxidation of water. Now the biofilm grows on the cathode . The energy-rich electrons are used by organisms to fixate carbon dioxide.
[MUSIC PLAYING]
Carbon dioxide enters the cathode compartment and fuses to the cathode. There, to harvest energy from the electrons, CO2 is fixed. And acetate is formed.
PRESENTER: All right. Let's have a look at our bio-battery. We buried the anode in our soil. Make sure there are no air bubbles in the soil. It has to be anaerobic. On top of the soil, we placed the cathode, which is in direct contact with the oxygen in the air.
Now let's look at another application of microbial fuel cell. Desalination can be achieved by inserting an extra compartment in between the anode and the cathode. A forward osmosis membrane is placed at the anode. This allows transport on both positively and negatively charged ions.
At the cathode, a cation exchange membrane is placed, which permits only transport of positively charged ions. Saltwater is flowing through this compartment while negatively charged ions move to the anode, and positively charged ions moved to the cathode.
To finalize our bio-battery and to see the energy production, we have to connect the cathodes to the anodes. the electricity is stored in a transformer and used to power an LED light.
[MUSIC PLAYING]
The next short video has Dr. Bruce Logan, a professor in the Civil Engineering Dept. at PSU providing a brief explanation on using these MFCs for wastewater treatment facilities (3:05).
PRESENTER: Clean water and electricity are essential for everyday life. But to get one, we often need the other. We generate electricity to purify water. And in many parts of the world, we use water to create energy.
In the United States, an average of 5% of the electricity we produce goes towards powering our water infrastructure. But what if we could use wastewater for energy? As it turns out, a decades' old technology known as microbial fuel cells can help extract the energy and wastewater to produce electricity.
BRUCE LOGAN: A microbial fuel cell is a device where we use bacteria to directly produce electrical current from something as simple as wastewater. Right now, we have wastewater treatment plants that consume electrical power. And we can imagine a time when these treatment plants are transformed into what we hope would be power plants.
PRESENTER: Deep in the sewers in wastewater treatment plants, there are billions of bacteria, or microbes, that break down organic matter to produce electrons. They surrender their electrons to oxygen molecules in exchange for energy. But in a microbial fuel cell, the electrons take a detour.
BRUCE LOGAN: This is a microbial fuel cell. It's really a very simple device. It's just a tube with electrodes on either side of that tube-- one which is sealed off, so the bacteria can't get at the oxygen, the other one which is exposed to oxygen.
The most important part of a microbial fuel cell are the microbes. The microbes grow on an electrode, which is oxygen-free so that they send off those electrons to an electrode rather than oxygen. The electrons flow through a circuit, so we extract that electrical current as electrical power.
PRESENTER: To complete the circuit, the electrons end up on the other side of the tube and combine with oxygen. The theory is simple. But putting it into practice is not so easy.
BRUCE LOGAN: We initially thought that the greatest challenge would be the bacteria. But as it turns out, it's actually everything but the bacteria that we've had the greatest challenges with. We have systems the size of this cube and maybe a little bit bigger, but we really haven't gone out and built 1,000-liter systems or 10,000-liter systems. And that's an engineering challenge that we need to address next.
PRESENTER: Despite these challenges, Logan is optimistic about microbial fuel cells.
BRUCE LOGAN: I am really excited about microbial fuel cells and these different technologies because it creates a truly sustainable way to produce energy and power our water infrastructure.
PRESENTER: In other words, if microbial fuel cells deliver on their promise, wastewater will be waste no longer.
[MUSIC PLAYING]
The last video, put together by Dr. Logan’s group at PSU, shows how to construct three different types of MFCs (4:26).
[MUSIC PLAYING]
PRESENTER: Here we have three different microbial fuel cell reactor architectures. In front, we have cube reactors. This is a single-bottle MFC reactor. And this is a double-bottle reactor, also known as an H-type reactor.
[MUSIC PLAYING]
Here we have a cube reactor disassembled. I just want to be able to show you all the parts before I put them back into the reactor itself.
This is the body of the reactor. It's a Lexan cube with a 28-milliliter volume anode chamber; two holes drilled in the top for refilling and emptying of the substrate.
This is the cathode end plate, and this is the cathode. This is actually an air cathode made out of carbon cloth.
This side has the four diffusion layers of PTFE. This side has carbon black and platinum catalyst. This is the side that actually faces the interior of the MFC.
This is the other end plate. There's many different nanomaterials that can be used. In this example, we are using a carbon brush fiber anode.
Now I will demonstrate assembly of a cube reactor.
First place the cathode in, the platinum-- carbon black side facing in. We use gaskets to make sure that there is no leakage and good connection between the platinum side and the current collector, which is a titanium wire.
The cube reactor is held together just with all thread and wing nuts using compression to keep all the places in-- all the pieces in place.
So the front's done. Apply a gasket, O-ring; the end plate gets slid on.
There you have it-- a cube reactor.
[MUSIC PLAYING]
So here we have a single-bottle reactor. This is very similar to the cube reactors. In this setup, we have a brush anode as well as another air cathode.
Now I'll demonstrate how to assemble a bottle reactor. So this is just a standard media bottle with an arm attached to it. Once again, replace the cathode with the platinum and carbon black side facing into the reactor and the PTF diffusion layer side facing towards the air.
So all we're doing is placing the cathode, current collector, O-ring-- this is just a cap that will be used for compression to keep the cathode in place-- using just a regular arm clamp. All that remains to be done is placing of the brush anode into the bottle.
And that is a single-bottle reactor.
[MUSIC PLAYING]
OK. Now I'll demonstrate the assembly of two two-bottle MFC reactor architecture, or an H-type reactor.
In this situation, the anode and cathode are both the same size, and we're using the same material for the anode and cathode, which is carbon paper.
And it's just been attached to a titanium wire as the current collector. And once again, this is being used as both the anode and the cathode.
In this situation, the cation exchange membrane is placed in between the two chambers.
Use a clamp. Tighten the clamp if necessary.
And you have a two-chamber microbial fuel cell reactor.
MFCs can also be used in food processing plants and breweries, as well as being implanted as biomedical devices. There are technical challenges to MFCs. They have relatively low power densities, which means they don’t generate much power. Therefore research continues to improve power densities. These devices have an incredible future but still need more research to be commercialized at a large scale.