Reading Assignment
- SECS, Chapter 5--Meteorology: the Many Facets of the Sky (section on Air Masses)
Case Goal: Develop a working concept of the atmosphere as a case study of a selective surface, interacting in concert with the Earth surface to contribute to the global energy budget. Then, move on to more specific reflective surfaces that we might use as a secondary shortwave resource (diffuse reflectors and specular reflectors) in designing SECS.
The Earth is a vast solar energy conversion system! The atmosphere encasing Earth's land and water mass is a collection of gases and particles that vary in pressure, temperature, and chemistry continuously. If we collectively imagine for just a moment that the atmosphere is a simple cover on top of our main absorber (the Earth), we can begin forming a concept of the atmosphere interacting with electromagnetic radiation across broad bands of wavelengths.
Now, let us consider the optical properties of a single material that reflects light for some bands and transmits or absorbs light for alternate bands, just like the atmosphere represented above. We call the surface of such a material a selective surface, because the light interaction occurs at the surface. A selective surface is non-reflective to some bands of light, while being reflective to other bands of light. The atmosphere is a case study for a selective covering surface between shortwave and longwave bands.
For any given wavelength or band of similar wavelengths, the following three simple phenomena will occur when light interacts with a material surface (where light is either being absorbed or emitted):
This means that each of the simple optical phenomena can be represented by fractions from 0 to 1, with the sum of these equating to 1.
For opaque materials, there is a relation between reflectivity and emissivity (the glow of an object) and between reflectivity and absorptivity.
- A surface that is highly reflective for a certain band of wavelengths is also a surface with a low emissivity.
- Meaning: reflective materials don't 'glow' effectively.
- In contrast, a surface that has low reflectivity for certain wavelengths of light will have a high emissivity.
- Meaning: non-reflective materials tend to 'glow' effectively.
We now know the relation for surfaces in optics called Kirchoff's Law of Radiation. When a surface is in thermal equilibrium with the surroundings, the emissivity is equal to its absorptivity at each wavelength ($\epsilon = \alpha$). This allows us to make the same relations among reflectivities and absorptivities.
- A surface that is highly reflective for a certain band of wavelengths is also a surface with a low absorptivity.
- Meaning: reflective materials don't absorb light effectively either.
- In contrast, a surface that has low reflectivity for certain wavelengths of light will have a high absorptivity.
- Meaning: non-reflective materials really do absorb light effectively.
We can show the ways in which the Earth-Atmosphere is selective in the relative properties of each to absorb, reflect, and transmit different bands of light.
Video: Solar Case Study (6:47)
Starting again with the image of the sun interacting with the atmosphere and the Earth. The atmosphere is a cover. It's a transparent cover relative to some wavelengths. And we'd like to use that as our quick diagram and case study.
So, we've got the sun that is emitting shortwave light. It is transparent in the shortwave, for the large part. That sunlight ultimately interacts with the Earth's surface, where it is absorbed. A portion of it is absorbed. And a portion of that light is reflected. And that reflected light ultimately is going to leave the atmosphere.
What we find out is that that amount of light that's leaving the atmosphere, whether it's reflected off the ground, or let's say, for example, it's sunlight that is being reflected off of clouds. We're still assessing that these (total) are going to be on the order of 70% to 75% of light. And that would be evaluated with a row of 0.7. We'd have-- from the balance, because the Earth is opaque, we'd have an alpha value of 0.3, so alpha of 0.3 for the surface of the Earth. That would make the transparency approximately 1 for the Earth's atmosphere.
Now that's part of the balance, and that's all the shortwave. But the Earth itself in this case study, again, has a given temperature. The temperature of the sun, and the temperature of the Earth, and the temperature of the atmosphere are all going to come to play in this.
Shortwave is coming from the source of the sun, because the sun's surface temperature is that 5,777 degrees Kelvin, or approximated as such. And the Earth is going to be emitting longwave light. The atmosphere is going to be emitting longwave light, only the atmosphere is actually two surfaces-- a top surface and, effectively, a bottom surface, and will be emitting up and down longwave radiation.
However, the Earth itself-- let's grab this guy and simplify it again-- the Earth itself is going to be emitting this longwave light. And a large part of that longwave light is going to be absorbed by the atmosphere. Some of that light, however, emitted by the Earth, is going to make its way through the atmosphere, and it's going to have a very low transparency-- we'll say approximately 0.1-- but some of that makes its way out.
And it makes its way out through what we call the sky window, or the atmospheric window. And that sky window is occurring in a specific band-- this is a selective surface of its own anywhere from 8 to 13 micrometers. We have a gap, a leaky window, where longwave radiation can escape.
So, if I went to grab this image that we've been looking at before, you're going to see a range, and we're going to dial into where is that sky window happening? So, somewhere right in here is a range that's lining up over in this area. And that range, if we look closely, is going to be-- let's bring that down here and carry that down.
That's going to be at about 10, 9, 8 nanometers. And the next one is going to be right here. Let's grab that guy. And he is going to be at out-- that's about 20, so half of that's 15, and approximately right there is about 13 or 14 micrometers.
So, again, from 8 to 13 micrometers, we have in this range right here and here, is our window where we're getting a percentage, actually, that looks like a higher percentage-- 15% to 30% of light transmitted through the Earth's atmosphere. On this side over here, we see that whole spectrum that is the solar spectrum from the sun. And it's in the small percentage in the UV, a bigger percentage in the visible band, and a very large percentage of light that's coming through the atmosphere that is in the infrared band. But ultimately, it all cuts off at about 1, 2, 3,000 nanometers. And actually, if we were to look closely, the atmosphere actually cuts off at about 2,500 nanometers.
Now, the other thing that we can look at in this diagram is, what are the sources of the walls of this atmosphere? I draw that arrow down-- oops-- if I draw that arrow down, I see that one of the absorbing gases is carbon dioxide. And on the other side of this, the main absorbing gas is going to be water vapor, water vapor and oxygen.
So, essentially, I have water vapor on this side. I have CO2 on this side, that are limiting the leakiness of the atmosphere to lose energy, to leak energy into space. And that's actually the valve mechanism that we'd tie into the greenhouse effect. So, if you increase CO2, you start moving the valve tighter. You heat up the atmosphere, you increase the water content, you squeeze things a little tighter. And you actually lose your leakiness, and you drive the atmosphere more and more warm.
OK, that's the end of our case study. Let's move on to the next.
We have already described shortwave (280-2500 nm at ground level) and longwave (>2500 nm) bands of irradiation incident upon a surface. The study of optics is that of light-matter interactions, regardless of wavelength. We know that materials like glass are semi-transparent in most of the shortwave band, and materials like pure aluminum are reflective in the shortwave band. We are not so familiar with the way that materials behave in the longwave band, and we often have trouble with materials that are opaque vs. semi-transparent.
Let's review the transmittance graphic that I introduced earlier. This plot has a logarithmic x-axis, so, we can count from 0.2 micrometers (200 nm) up to 1 micrometer wavelengths in increments of 0.1 micrometer. Then, we count from 1 micrometer to 10 micrometers in increments of 1 micrometer, and so on...
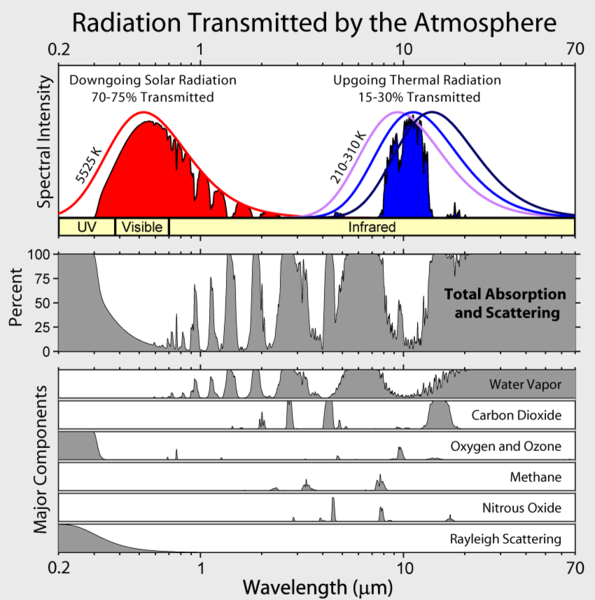
We see that from about 0.3 micrometers to about 2.5 micrometers, there is a significant amount of white showing on the plot of the Total Absorption and Scattering, meaning that the absorption and reflection of visible light by the atmosphere is relatively small. In other words, the atmosphere transmits 70-75% of the sun's shortwave light from the top down to the Earth's surface. Along the way, clouds can back-scatter (reflect) some of the visible light into space. In times and places where transmitted sunlight reaches the Earth's surface, then the land, oceans, deserts, grasses, and trees, etc., reflect some of the surface-incident shortwave light back into space (again, with limited absorption along the way).
We also see that from 8-13 micrometers, there is an atmospheric window where the longwave band light is not absorbed or scattered. This is the way that the Earth and the Atmosphere can "leak" energy back into space. If you like, go back to take a look at a similar figure from Granqvist. From which side of the sky window is water vapor absorbing the longwave light, and from which side is CO2?
Using this example, when incident light (irradiance) is not absorbed or transmitted through the surface and bulk of a material, it is reflected by the surface (the fraction of reflection is called the albedo).
