9.2. Battery storage
Batteries are commonly used to store electric energy generated by off-grid renewable energy systems, and also to mitigate the sharp fluctuations of power for on-grid systems. While there are many different types of battery technologies, some are more applicable to utility scale energy storage than others. Applicability to large systems depends on such factors as cost of materials, ability to scale up with no ill effects or performance loss, and design and operation mode.
Some well-known examples of battery types used as stationary storage system for PV solar are listed in Table 9.1
| Technology (battery type) | Power subsystem cost $/kW | Energy storage subsystem cost $/kW | Charge-discharge efficiency % | Cycles |
|---|---|---|---|---|
| Advanced lead-acid | 400 | 330 | 80 | 2,000 |
| Sodium/sulfur | 350 | 350 | 75 | 3,000 |
| Lead-acid with carbon enhanced electrodes | 400 | 330 | 75 | 20,000 |
| Zinc/bromine | 400 | 400 | 70 | 3,000 |
| Vanadium redox | 400 | 600 | 65 | 5,000 |
| Li-ion (large) | 400 | 600 | 85 | 4,000 |
| Flywheels (high-speed composite) | 600 | 1,600 | 95 | 25,000 |
| Super capacitors | 500 | 10,000 | 95 | 25,000 |
Note: The costs in the table are based on standard assumptions for the applications and technologies considered, and on expert opinion. They are meant to be used for comparative purposes. The actual costs of any storage system depend on many factors and the assumptions and the means of calculating some of the values are subjective and continue to be debated, even among experts in the field (Sandia National Laboratories).
For quite a while, lead-acid batteries have been the first choice for off-grid PV applications. This lead-acid battery technology has been around since the 19th century and, historically, service providers have more knowledge and tools to deal with those systems. But, despite their long existence and widespread use, lead-acid batteries remain one of the lowest energy-to-weight and energy-to-volume battery designs, which means they are too big and heavy for the amount of energy they provide. This technology is inexpensive and reliable, and it may be a while before it is replaced by more advanced types on a wide scale.
The following reading provides more information on the battery storage types and lead-based batteries, specifically.
Reading Assignment:
Book Chapter: Foster, A., Chassemi, M., and Cota, A., Solar Energy. Renewable Energy and the Environment. CRC Press, 2010. Chapter 11. Energy Storage, pp. 265-293. (See E-Reserves via the Library Resources tab.)
Li-ion battery technology
Li-ion battery is one of the rapidly advancing technologies preferred for employment in conjunction with solar systems due to high storage capacity, high charging rates, light weight, and relatively long service life. However, the technology cost is still high and can be a limitation on the utility scale. Some of the very attractive features of Li-ion batteries are high power output and high charge-discharge efficiency. They can also withstand more charge-discharge cycles than lead-acid batteries.
The principle of operation of the Li-ion battery is discussed below.
A schematic representation of a generic Li-ion battery is given in Figure 9.1. Roughly, Li-ion cell consists of three layers: electrode 1 (cathode) plate (usually lithium cobalt oxide), electrode 2 (anode) plate (usually carbon), and a separator. The electrodes inside the battery are submerged in an electrolyte, which provides for Li+ ion transfer between the anode and cathode. The electrolyte is typically a lithium salt in an organic solvent.
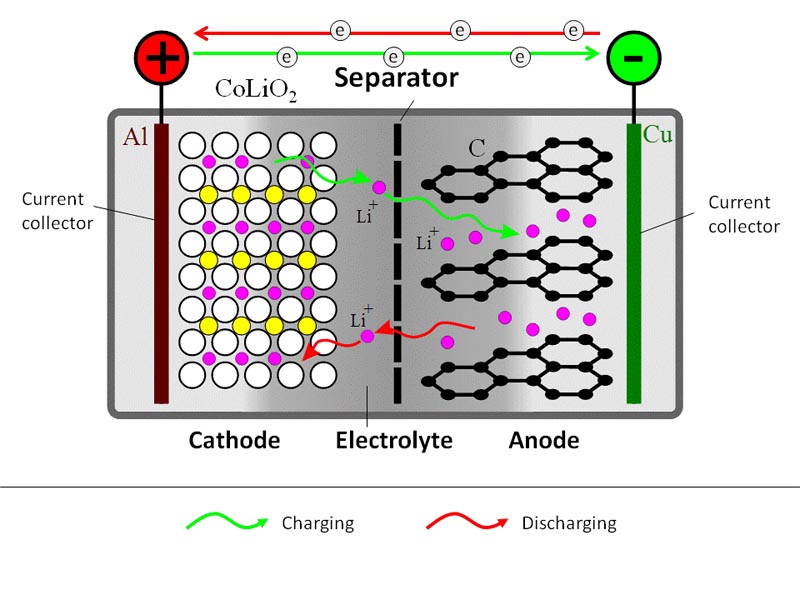
During the charging process, a DC current is used to withdraw Li+ ions from the cathode and to partially oxidize the cathode compound:
LiCoO2 → Li1-xCoO2 + xLi+ + xe–
The released Li+ ions migrate through electrolyte towards the anode, where they become absorbed in the porous carbon structure:
xLi+ + xe– + C6 → LixC6
At the same time, electrons travel through the external circuit (electrolyte is not electron conductive).
During the battery discharge, the reverse process takes place. Li+ ions spontaneously return to the cathode, where electrochemical reduction occurs.
Limitations of the Li-ion batteries are rooted in the material properties.
For example, the LiCoO2 ⇔ Li1-xCoO2 conversion is only reversible with x<0.5, which limits the depth of the charge-discharge cycle. But with a wider variety of materials available, research is underway to develop new generations of Li-ion batteries.
For example, take a look at the Sigma Aldrich website, which lists multiple alternatives for cathode, anode, electrolyte, and solvents.
| Advantages | Limitations |
|---|---|
| 1. Relatively high energy density and potential of finding even better formulations. | 1. Circuit protection needed to avoid damaging high voltage / current. |
| 2. No need for priming - new battery is ready to operate. | 2. Aging - battery gradually loses its capacity even if not in use. |
| 3. Low self-discharge (compared to other types of batteries). | 3. Toxic chemicals are subject to regulations. |
| 4. Low maintenance. | 4. High cost of materials and manufacturing process. |
| 5. Capability to generate high current / power. | 5. Technology is not fully mature; varying components and chemicals. |
Supplemental reading on the status of Li-ion battery technology:
Goodenough, J.B. and Park, K.S., The Li-Ion Rechargeable Battery: A Perspective, J. Am. Chem. Soc., 2013, 135 (4), pp 1167–1176.
Etacheri, V., Marom, R., Elazari, R., Salitra, G., and Aurbach, D., Challenges in the development of advanced Li-ion batteries: a review, Energy & Environmental Science, 2011 (9), 3243-3262.
Flow Batteries
Flow batteries, unlike solid-state batteries, have their chemical components dissolved in liquid solutions, which can be pumped through the electrodes in a flow. If you are familiar with the concept of fuel cell, it is something similar in principle of operation, although it is still a closed loop system. A flow battery cell itself can be small, while the solutions can be contained in external storages. One of the advantages of the flow batteries is almost instant replacement of the electrolyte liquid, thus eliminating any gradient or concentration fluctuations at the electrodes. The main difference between the conventional batteries and flow batteries is that the energy is typically stored in the liquid phase in flow batteries. So, increasing the size of the storage tanks for the liquids allows easy scale-up of the battery to match a specific application.
Zinc-bromine flow battery storage
Zinc-bromine battery is a type of hybrid flow battery. It uses zinc bromine as the working solution, which is stored in two compartments, separated by a porous membrane. One compartment has a negative zinc electrode and the other compartment has a positive bromide electrode. During charge, supplied electricity (e.g., from a solar conversion system) is used to electroplate metallic zinc (Zn) on the negative electrode, while bromine (Br2) is generated on the positive electrode. During discharge, the opposite process occurs: Zn is dissolved to form Zn2+ ions in solutions, and bromine is converted back to bromide ions (Br-).
Here are the electrochemical reactions involved in this process:
Zn2+ + 2e- → Zn(s) - Reduction of zinc during battery charging
2Br- → Br2(aq) + 2e- - Oxidation of bromine during battery charging
The overall reaction is therefore:
Zn2+ + 2Br-⇔ Zn(s) + Br2(aq)
This reaction proceeds to the right on charging and to the left on discharging. The standard electrode potential for the overall reaction is 1.85 V, which is the maximum theoretical voltage that can be expected from a single cell. The battery cells are stacked to increase the overall storage capacity of the system.
The battery compartments are made of inert plastic. Unlike common batteries, which store electrolyte within the reaction chamber, zinc-bromine batteries have solution storage in the external tanks, from where it is circulated through the electrodes (flow battery type). The external bromide solution storage also helps maintaining required concentration of bromide throughout the reaction cycle.
This technology has been commercialized by ZBB EnerStore company, which engineered zinc-bromine batteries into 50 kWh modules, scalable up to bigger storage systems. Each module is a stand-alone system that includes all necessary software and hardware. Some advantages of this technology include high-energy density (75-85 Wh/kg), stability, i.e., good resistance to performance degradation, ability to operate at full output within a wide temperature range. Unlike most batteries, ZBB EnerStore batteries use non-reacting electrodes (i.e., electrodes are not reactants, but simply are substrates for reactions to take place), which helps minimize loss of performance from repeated cycling.
Watch this video (5:19 minute) for a demo of ZBB EnerStore solution for zinc bromide battery technology:
Video: ZBB EnerStore (5:19)
PRESENTER: Introducing ZBB EnerStore, ZBB Energy's third generation flow battery. ZBB has completely re-engineered its zinc bromide flow battery to be the most advanced and cost effective energy storage device for distributed energy projects, whether on or off the grid. The new design is easy to maintain and operates indoors or outdoors in the widest ambient temperature range of any flow battery, so there are no additional costs associated with constructing and maintaining a climate-controlled building. ZBB EnerStore batteries are self-contained, modular units and are easy to transport, so we can deliver an expandable solution that is virtually plug and play at your site with no on-site wiring needed. Although there are many different energy storage options to consider when compared to lead acid batteries, ZBB's flow batteries have a higher energy density, the lowest cost of ownership over a 20-year lifespan and are the most effective way to maximize the use of renewables. By utilizing energy storage with renewable power sources, diesel gensets can be configured to operate in backup mode only, reducing run time and fuel consumption, and you don't need to worry about the complexities of federal emission compliance. ZBB flow batteries are also "black start" capable. Most other energy storage systems need the grid to work. The ZBB EnerStore flow battery is well-suited for a variety of commercial applications with or without renewable energy sources and is readily configurable and scalable.
The ZBB EnerStore battery is built in 50 kilowatt hour modules and expandable to 500kWh in a singular enclosure. With multiple enclosures linking together, it can contain up to 2 megawatt hours or more on a single point of connection. Each battery module is comprised of eight cell stacks with 60 cells each, DC to DC converters, a self-regulating heat exchanger, electrolyte storage tanks, and built-in secondary spill containment. With ZBB's innovative, integrated DC bus architecture and converters on each module, there are no voltage limitations or concerns about system design and operation. In addition, ZBB's flow batteries operate without any special ventilation requirements. Since flow batteries are made with inert materials, they are safe to install. Electrolytes are never handled or replaced, only the cell stacks are changed out over the course of its life cycle. ZBB's flow batteries are environmentally friendly, made with highly-recyclable materials and recoverable at the end of their service life. Now let's take a look at how the zinc bromide battery works. ZBB's flow battery technology is based on the reaction between two readily available chemicals, zinc and bromine. The battery consists of a zinc anode and a bromide cathode, separated by a microporous separator. The aqueous solution of zinc bromide is circulated through the two compartments of the cell from two separate reservoirs.
During charge, electricity causes a reaction with the zinc bromide solution to electroplate zinc and form bromine on the battery electrodes. The reaction is reversed during discharge. The zinc and bromine reacts electrochemically to produce electricity while reforming the zinc bromide solution. Circulation pumps are used to allow the zinc and bromide to flow continuously throughout the cell stacks. While the electrolyte is flowing, an onboard computer calculates the state of charge of the battery module and directs when energy should be stored or if the stored energy should be discharged. An inline heat exchanger regulates the temperature of the battery module and keeps it within the standard operating range regardless of ambient temperature or rate of charge and discharge operation. The ZBB EnerStore can be left indefinitely at any state of charge, from 100% to completely discharged. The ZBB EnerStore battery is your answer for capturing multiple value streams, from time shifting, to firming of renewables or load management, to system backup. It's the best choice over a 20-year cost of ownership. Using EnerStore as part of ZBB's integrated management platform provides a continuous supply of energy and optimizes all of your interconnected resources, no matter your energy source or connection to the grid. ZBB, optimizing energy availability.
Reading Assignment
Learn more on Zn-Br battery technology:
Book chapter: Butler, P.S., Eidler, P.A., Grimes, P.G., Klassen, S.E., and Miles, R.C., Zinc/Bromine Batteries, in Advanced Battery Systems, Sandia National Laboratories, pp 31.1-37.15. (See E-Reserves via the Library Resources tab).
Vanadium Redox Flow Batteries
This type of battery utilizes the multiple redox states of vanadium (V) in its charge-discharge cycles. Vanadium is present in the dissolved form in the sulfuric acid medium, and because it is all-vanadium system, this type of battery is not susceptible to performance loss due to cross contamination.
During charging, the following half-reactions occur in two separate compartments of the battery:
V3+ + e– → V2+
VO2+ + H2O → VO2+ +2H+ + e–
Electrons are supplied from the solar energy conversion system as DC current onto non-reacting electrode dipped in the V3+ solution. As a result, V3+ is reduced to V2+. At the same time in the other compartment, vanadium (IV) species VO2+ is oxidized to vanadium (V) species VO2+, releasing the electron. On discharging, these reactions are reversed.
The summary process is expressed through the following reaction:
VO2+ + V3+ + H2O ⇔ V2+ + VO2+ + 2H+
The total voltage generated by a single vanadium redox flow battery is around 1.25 V in ideal case.
The main benefits of the vanadium redox flow batteries ability to go through "unlimited" number of cycles; they have a long lifespan (>20 years), quick charging, and high efficiency of the charge-discharge cycle (~80%). They are also more environmentally friendly in terms of component toxicity than many other types of batteries.
Reading Assignment
The following sources will help you to better understand the technical details of the vanadium redox batteries, as well as its challenges.
DOE Fact Sheet: Wang, E., Vanadium Redox Flow Batteries, U.S. Department of Energy, Energy Storage Program, 2012.
The vanadium redox flow battery technology is potentially suitable for extra-large utility scale applications. For example, the 200 MW VRB battery facility in Dalian, China, is expected to significantly increase the stability of the electric grid by supplying power during peak hours and emergency black-starts. Development of such a mega facility was enabled by its co-location with the VFB cell manufacturing factory, which is tapping into local vanadium resources. The Dalian battery is expected to become operational in 2020. Nearby wind power facilities have been forced to curtail electricity production – this battery facility hopes to reduce curtailing significantly.
Probing question
Will the Dalian energy storage facility become truly the largest battery in the world when brought online? What is the capacity of the largest Li-ion Battery storage built to date, and how does that compare?
Additional resources:
Review paper: Blanc, C. and Rufer, A., Understanding the Vanadium Redox Flow Batteries, in Paths to Sustainable Energy, Dr. Artie Ng (Ed.), ISBN: 978-953-307-401-6. pp. 333-337(Access the article in Canvas).
This paper is quite technical as it describes different models used to analyze the performance of the vanadium redox flow batteries. Read sections 1 and 2, which describe the electrochemical principles behind the battery operation. Reading further sections may be useful if you have a special interest in this topic, but is not required.
