Aromatic Hydrocarbons
Aromatic hydrocarbons are an important series of hydrocarbons found in almost every petroleum mixture from any part of the world. Aromatics are cyclic but unsaturated hydrocarbons with alternating double bonds (Figure 1.12). The simplest aromatic hydrocarbon is benzene (C6H6). The name “aromatic” refers to the fact that such hydrocarbons are commonly fragrant compounds. Although benzene has three carbon-carbon double bonds, it has a unique arrangement of electrons with resonance structures of the double bonds (aromaticity) that allow benzene to be relatively stable. However, benzene is known to be a cancer-inducing compound. For this reason, the amount of benzene allowed in petroleum products such as gasoline or fuel oil is limited by government regulations in many countries. Under standard conditions, benzene, toluene, and xylene are in liquid form whereas higher aromatics such as naphthalene occur as solids in isolation, but dissolve to form a liquid solution with simple aromatics.
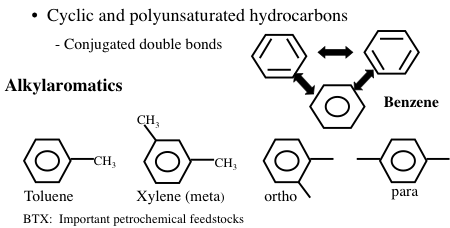
Image Shows Cyclic and polyunsaturated hydrocarbons with conjugated double bonds. Specifically:
Benzene: six carbon ring with no side chains
Alkylaromatics:
-Toluene: benzene with a methyl group on carbon 1
-Xylene (meta): benzene with a methyl group on carbons 1 & 3
-Ortho: benzene with a methyl group on carbon 1&2
-Para: benzene with a methyl group on carbon 1&4
Knowledge Check
What constitutes the white crystals of moth balls?
ANSWER: Napthalene! Naphthalene is an effective moth killer because it sublimes (forms a vapor from a solid without going through a liquid state) at room temperature.