Catalytic Reformer
Catalytic reforming converts low-octane straight run naphtha fractions (particularly heavy naphtha that is rich in naphthenes) into a high-octane, low-sulfur reformate, which is a major blending product for gasoline (Figure 3.3). The most valuable byproduct from catalytic reforming is hydrogen, which is needed in refineries with increasing demand for hydrotreating and hydrocracking processes. Most reforming catalysts contain platinum supported on alumina, and some may contain additional metals such as rhenium and tin in bi-, or tri-metallic catalyst formulations. Early reforming processes were called platforming in reference to reforming with a platinum catalyst. In most cases for catalytic reforming, the naphtha feedstock needs to be hydrotreated before reforming, to protect the platinum catalyst from poisoning by sulfur or nitrogen species. The principal reactions in catalytic reforming include dehydrogenation of naphthenes to aromatics (with significant quantity of hydrogen as byproduct) and cracking/isomerization of n-paraffins into i-paraffins. The principal product from catalytic reforming is called reformate, consisting of C4 to C10 hydrocarbons. Reformate has a high octane number because of high concentration of aromatic compounds (benzene, toluene, and xylene) produced from naphthenes. With the more stringent requirements on benzene and total aromatics limit in US and Europe (less than 1% benzene, 15% total aromatics), the amount of reformate that can be used in gasoline blending has been limited, but the function of catalytic reforming as the only internal source of hydrogen continues to be important for refineries.
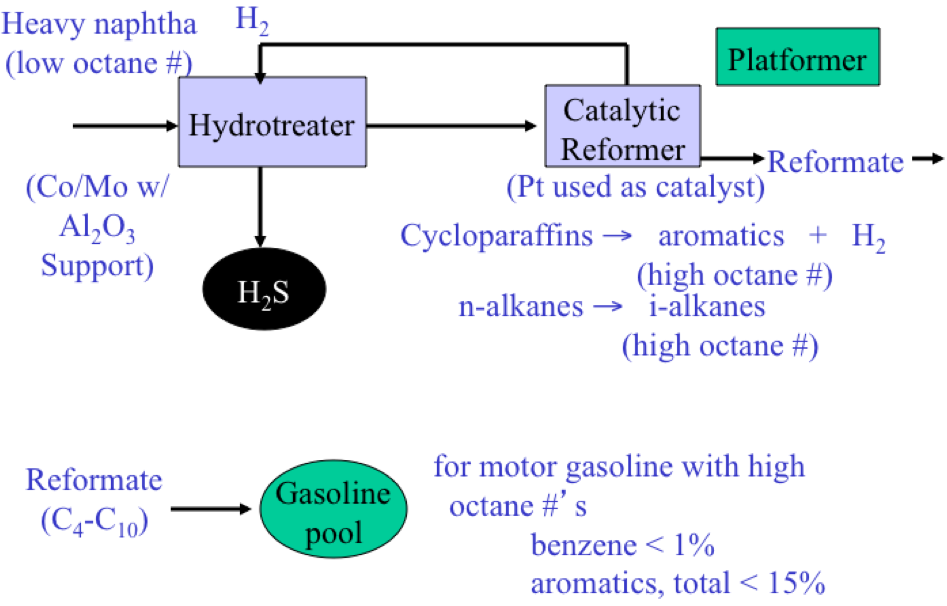
Catalytic reforming schematic.
Heavy naphtha (low octane #) along with Co/Mo with Al2O3 support enter the hydrotreater. From there it goes to the catalytic reformer where Pt is used as a catalyst to turn cycloparaffins into aromatics + H2 (high octane #) and n-alkanes into i-alkanes (high octane #). H₂ goes back into the hydrotreater and is removed as H2S. Products from the catalytic reformer are called reformate and are added to the gasoline pool.
Also noted on the diagram is that for motor gasoline with high octane #’s benzene accounts for less than 1% while aromatics, total are <15%