Chemistry of Thermal Cracking
Thermal cracking produces shorter straight chain alkanes from longer straight chains found in gas oils or other crude oil fractions. Free radicals (reactive species with unpaired electrons, but no electronic charge) are the active species that govern thermal cracking reactions. Because of the free radical chemistry, thermal cracking of gas oil would produce gasoline with relatively low octane numbers, as will be discussed later in this section.
Figure 6.1 lists the three steps of free radical chain reactions as initiation, propagation, and termination. In the figure, R-H represents a paraffin chain which can be expanded such as (H3-(CH2)n – H) where n represents the number of carbon atoms in the alkane. In other words, R represents a radical with an unpaired electron that becomes an alkane (R-H) when combined with a hydrogen atom. A Hydrogen atom with one proton and one electron is the simplest radical.
The free radical chain reaction starts with breaking the weakest C-C bond in the reactant alkane (R-H) to form two free radicals R1 and R2, each with one unpaired electron resulting from the homolysis of the C-C bond (initiation). Once formed by the initiation step, each free radical can go through two different propagation reactions:
- Hydrogen abstraction
- Beta (β) scission
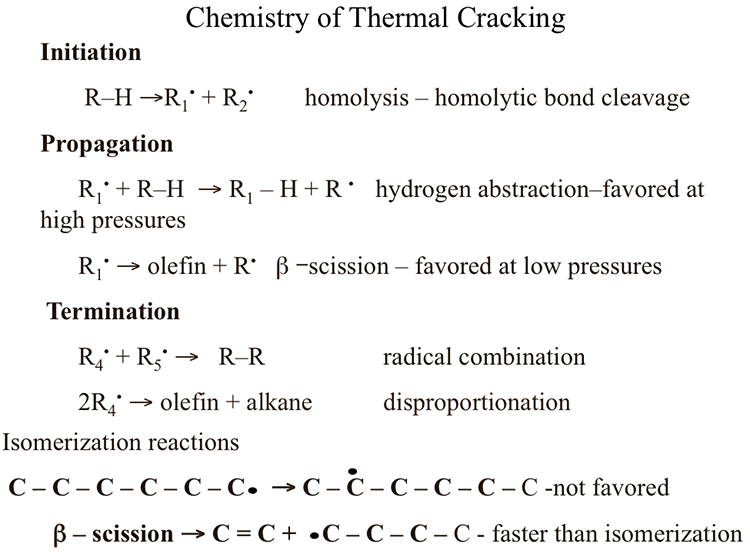
Initiation
R-H → R1• +R2•. Homolysis -homolytic bond cleavage
Propagation
R1• + R-H → R1-H + R•. Hydrogen abstraction – favored at high pressures
R1• → olefin + R•. β Scission – Favored at low pressures
Termination
R4• + R5• → R-R. Radical combinations
2R4• → olefin + alkane. Disproportionation
Isomerization reactions
C – C – C – C – C – C• → C – C• – C – C – C – C. Not favored
C = C + •C – C – C – C. β Scission, faster than isomerization
In a hydrogen abstraction reaction, for example, the radical R1 removes (abstracts) a hydrogen atom from an alkane (R-H) to produce a shorter chain alkane product (R1-H) and a new radical R (hydrogen abstraction), thus propagating the free radical chain. Alternatively, the radical R1, (or R2) can go through a β scission reaction to produce an olefin (ethylene) as a product, and a radical R to propagate the chain. The β scission refers to the breaking of the covalent bond in the β position relative to the position of the unpaired electron, as shown below:
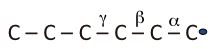
Note that the initiation step produces two free radicals, the propagation step produces a reaction product and one radical to continue the chain. The last step in the chain reaction, the termination step, removes two radicals to produce one or two stable compounds depending on the termination reaction, as seen in Figure 6.1. The principal end result of the free radical chain reactions in thermal cracking is to produce from long chain alkanes shorter-chain alkanes, light olefins, and some aromatic compounds. One important feature of free radical reactions is that isomerization reactions, e.g., shifting of the unpaired electron site from an edge atom of a molecule to the interior atoms (as shown in Figure 6.1), are not favored reactions. In other words, isomerization reactions take place at a slower rate than other propagation reactions, e.g., β scission reaction. The critical importance of this observation is that the thermal cracking reactions produce shorter straight-chain alkanes and olefins without any significant formation of branched-chain (or iso-alkanes). This is the reason why catalytic cracking processes have virtually replaced thermal cracking processes to produce high octane number gasoline, as will be discussed in the next section on catalytic cracking.
Knowledge Check
Please take a few minutes to answer the question below. Click "Check My Answer" to see some feedback.
Check your understanding!
Question: Why is hydrogen abstraction reaction favored over β scission at high pressures?
ANSWER: Hydrogen abstraction is a bimolecular reaction that is favored at high pressures over a monomolecular reaction (β scission).