Desirable Chemical Reactions
Figure 8.2 illustrates more specifically the desirable chemical reactions of catalytic reforming, including:
- dehydrogenation of naphthenes to aromatics,
- dehydroisomerization of alkyl-C5-naphthenes,
- dehydrocyclization of n-paraffins to aromatics, and
- isomerization of n-alkanes to i-alkanes.
All of these reactions significantly increase the octane number (research octane number [RON] from 75 to 110 in Reaction 1, from 91 through 83 [cyclohexane] to 100 in Reaction 2, from 0 to 110 in Reaction 3, and from –19 to 90 in Reaction 4).
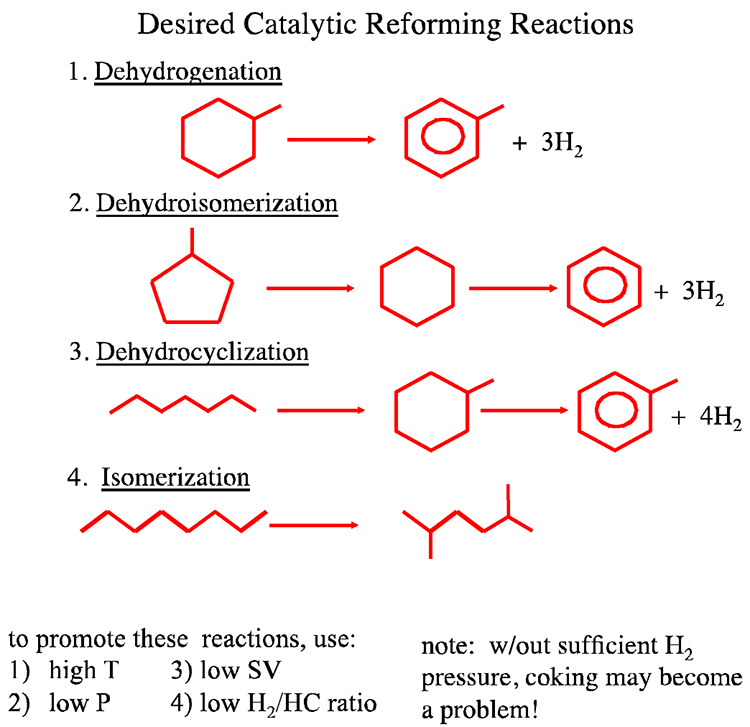
Reaction conditions that promote the desirable reactions are also listed in Figure 8.2. As can be seen in Figure 8.2, aromatic compounds and large quantities of by-product H2 are produced in the highly endothermic Reactions 1–3. High temperatures, low hydrogen pressures, low space velocity (SV), and low H2/HC ratio strongly promote the conversion in Reaction 1-3. Although maintaining a low hydrogen pressure is needed for promoting equilibrium conversion in Reactions 1-3, it is, however, necessary to maintain a sufficiently high hydrogen pressure in the reactors to inhibit coke deposition on the catalyst surfaces.