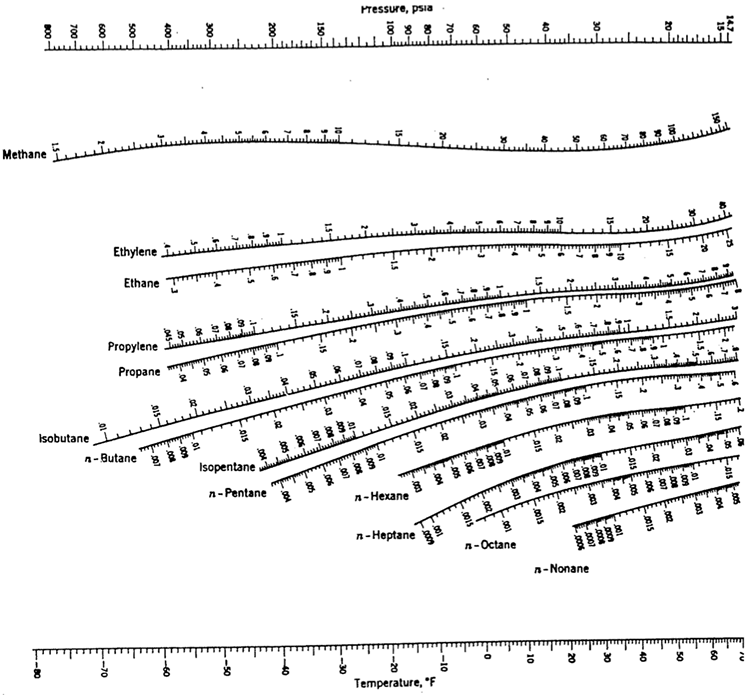
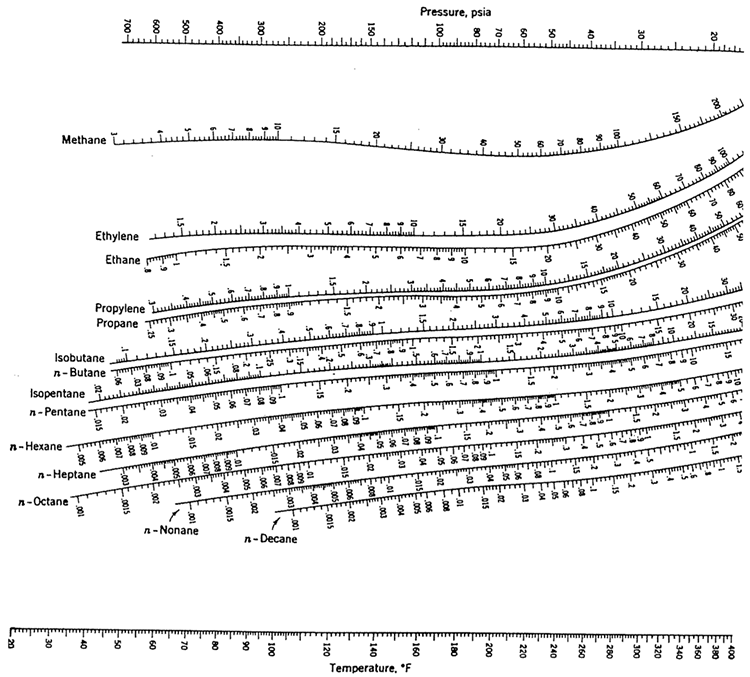
Exercise
Using the problem definition given in Figure 5.3, calculate n-C4 flow rate in the distillate product, if the debutanizer column has 18 (actual) plates.
Constraint:
The concentration of i- C5 should be <1 mole% of total C4’s and lighter compounds in the distillate product.
Given:
Column efficiency =75%, Reflux correction factor =1.5
Mean tower conditions: 210°F and 110 psig
Select appropriate light and heavy keys, and use the K values for the corresponding key compounds using the chart given in Figure 5.4 at the mean tower conditions.
Instructions for Submitting Your Answers:
Once you have calculated your answer, post it in the Exercises Dropbox inside the Lesson 5 folder in Canvas.
If possible, submit a Microsoft Word, or Excel document, showing all the steps in your calculations, indicate the K values you read from the nomograms, and assumptions, if any. You may submit scanned images or clear handwritten pages as a pdf that is less than 2 MB in size.
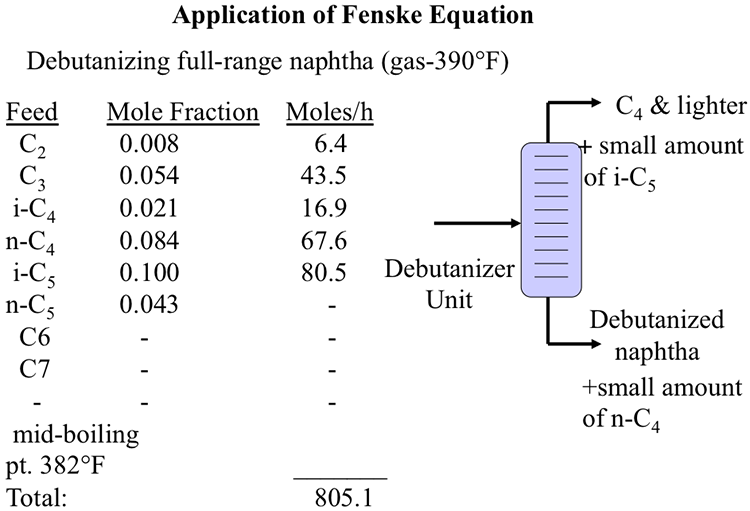
Application of Fenske Equation
Image shows a debutanizer unit which splits full-range naphtha (gas – 390*) into C4 & lighter (with small amounts of i-C5) and debutanized naphtha (and a small amount of n-C4)
It also notes that the mid-boiling point is 382*F and that the total is 805.1
| Feed | Mole Fraction | Moles/h |
|---|---|---|
| C2 | 0.008 | 6.4 |
| C3 | 0.054 | 43.5 |
| i-C4 | 0.021 | 16.9 |
| C4 | 0.084 | 67.6 |
| i-C5 | 0.1 | 80.5 |
| C5 | 0.043 | - |
| C6 | - | - |
| C7 | - | - |