HDS catalysts
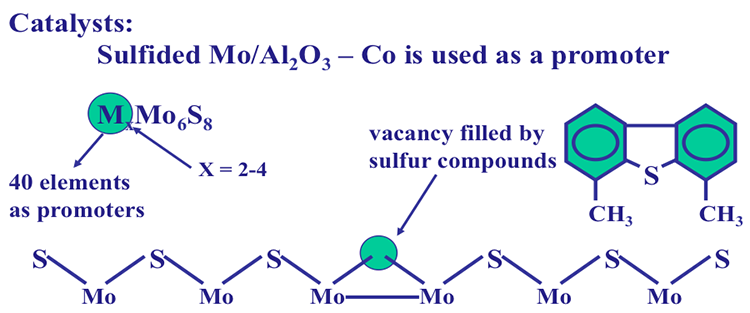
The HDS catalysts consist of sulfided molybdenum supported on Al2O3. Sulfided molybdenum surfaces have vacancies (with missing S atom in the sequence) that act as active sites on the catalyst surface (Figure 9.5). In addition to Mo, HDS catalysts also contain other metals, such as cobalt (Co) as promoters, the main function of which is believed to dissociate H2 to atomic species that readily react with S. The HDS mechanism involves inviting the sulfur atom at the HDS target to sit in the vacancy and once the S is in the vacancy, dispatching H atoms to clip C-S atoms to make H2S that will leave the vacancy and make it available for the next action. You can see that this scenario would get into trouble if S cannot sit in the vacancy, as would happen with the compound 4,6 dimethyldibenzothiophene (Figure 9.4 on the previous page and Figure 9.5 below). This calls for saturating the aromatic rings to twist the methyl groups aside to make the S atom fit in the vacancy to be taken away as H2S. The list below lists the relative HDS reactivities of methylated DBT compounds relative to HDS of unsubstituted DBT.
Reactivity in HDS (k methylated DBT/k DBT)
- 4, 6 dimethylIDBT → 0.1
- 3,7 dimethylIDBT → 1.5
- 2,8 dimethylIDBT → 2.6
- 4-methyl-DBT → 0.2
One can see above that the HDS reactivity of 4,6 dimethyldibenzothiophene (4,6 dimethylDBT) is one-tenth of unsubstituted dibenzothiophene (DBT), because of the shielding of the S atom by methyl groups as discussed above. Moving the methyl groups away from the sulfur atom to 3,7 positions (See Figure 9.4) increases the HDS reactivity 15- fold to 1.5 times of DBT. Interestingly, having methyl groups away from the S atom (as in 3,7 and 2,8 positions) increases the HDS reactivity relative to that of DBT. Methyl substitution on DBT away from the S atom increases the HDS reactivity by promoting adsorption of the compounds on the catalyst surface and may weaken the C-S bonds on DMDBTs. In contrast, even one methyl group shielding the S atom (4-methyldibenzothiphene in list above) reduces the reactivity of the methylatedDBT to one-fifth of the reactivity of DBT.