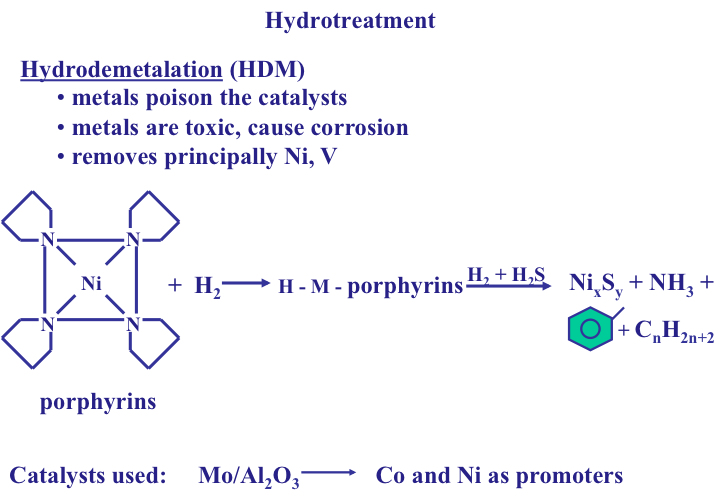
Hydrodemetallation
Similar to HDS and HDN, hydrodemetallation (HDM) is carried out: 1) as a pretreatment operation to remove metals (mostly nickel and vanadium) to protect the catalysts in subsequent conversion reactions, and 2) for removing metals in petroleum fuels to prevent corrosion in furnaces and toxic emissions from combustion engines. As mentioned in Lesson 1, metals (Ni, V) are mostly incorporated in the cage-like structures of highly stable organometallic compounds called porphyrins. As seen in Figure 9.8, to remove the metals, the cage structure of porphryins needs to be broken up. Similar to HDS and HDN, hydrotreatment on a Mo/Al2O3 catalyst first breaks up the chemical bonds in porphyrins to free the metals. The metals are then reacted with H2S and precipitated as metal sulfides, e.g., NixSy, on catalyst surfaces. Note that this is different from HDS and HDN where the heteroatoms are removed as gas (H2S and NH3, respectively). [Think: What would be the significance of removing metals as metal sulfides as far as the morphology of the HDM catalysts is concerned? Save your answer for the Self-Check question later in this lesson. ] Metals Ni and V deposited on the catalysts can be recovered by post treatment of the used catalysts.
In addition to HDS, HDM, and HDN, there may be a need for hydrodeoxygenation and hydrodehalogenation processes to remove O, or Cl in petroleum products [1].
[1] Petroleum Refining, by J. H. Gary, G. E. Handwerk, M. J. Kaiser, 5th Edition, CRC Press NY, 2007, Chapter 9, Hydrotreatment, pp. 195-203.