Hydrodesulfurization
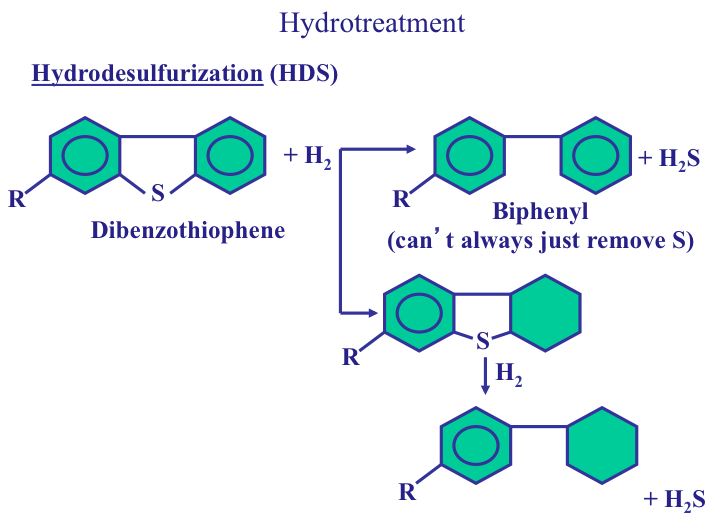
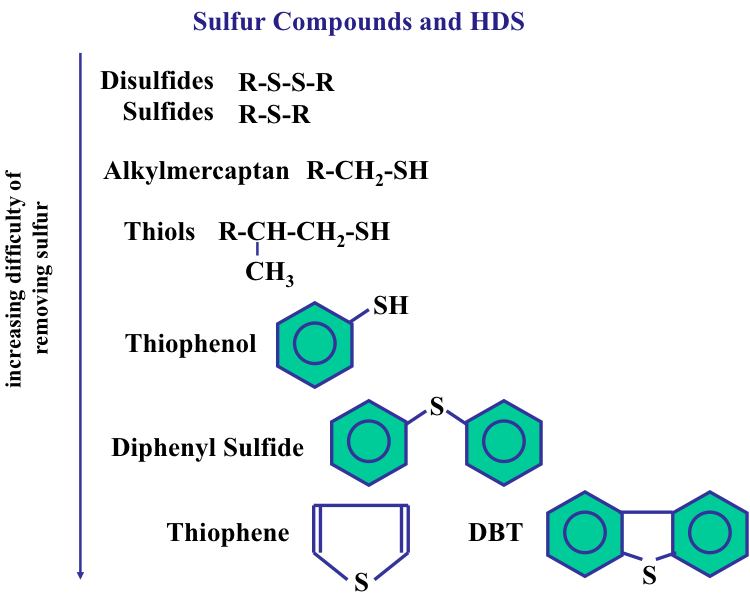
Desulfurization of fuels is commonly achieved by catalytic hydrodesulfurization (HDS), in which the organic sulfur species are converted to H2S and the corresponding hydrocarbon, as in the following reaction: R-SH + H2 → R-H + H2S. Here R represents an alkyl group, such as methyl (CH3–), or ethyl (C2H5–). Figure 9.2 shows the type and relative reactivity of different sulfur species in HDS reactions. The reactivity of R-SH (mercaptan, or thiol) compounds is higher than that of disulfides (R-S-S-R). The H2S is easily removed from the desulfurized oil by absorption in a gas treatment unit and subsequently converted to elemental sulfur by the Claus process, as will be discussed in Lesson 10.
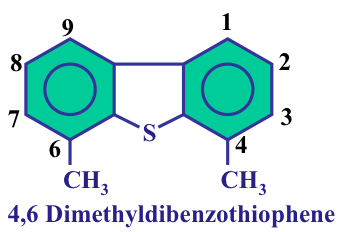
Some HDS reactions are shown in Figure 9.3. Note that the objective of hydrotreatment reactions is to take the heteroatom out with minimum hydrogen consumption. The ideal scenario is to get the hydrogen atoms to break the carbon-sulfur bonds and to remove sulfur as H2S. This may not always be possible because of problems with the accessibility of sulfur atoms to hydrogen atoms. Sulfur ring compounds such as methylated dibenzothiophenes have the lowest reactivity in HDS reactions because they are planar compounds, and in some methylated DBTs the S atom is shielded by the methyl groups (See Figure 9.4). Removing sulfur from these compounds requires more hydrogen consumption in order to saturate the aromatic rings in dibenzothiophene (DBT) to form non-planar compounds. Remember that, as opposed to aromatic compounds, cycloalkanes are not planar compounds. Therefore, saturation of the aromatic rings in methylated DBTs eliminates the steric hindrance (shielding) of methyl groups to make the S atom accessible to active sites on the catalyst surface for removal as H2S. The geometry of this proposition is better understood when one looks at the configuration of active sites on the HDS catalysts shown in Figure 9.5.