Thermal Reactivity Considerations in Processing
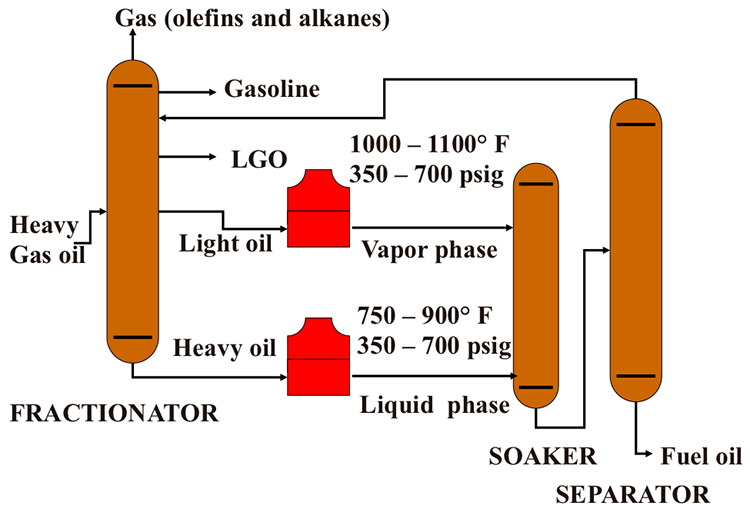
Management of thermal reactivity is important in thermal cracking processes for optimum conversion of the feeds with a wide boiling range. Figure 6.2 shows an example of a thermal cracking process to convert a heavy gas oil fraction primarily to light gas oil (LGO). Typically, lighter products (gasoline and gas) and the heavier product (fuel oil) are considered by-products in this process. A heavy gas oil feedstock with a wide boiling range is fed to a fractionator to separate the feed into a light oil and a heavy oil fraction, depending on the desired cut points. Light oil (which would have a relatively low thermal reactivity) is heated in a separate furnace to higher temperatures so that the cracking takes place in a vapor phase. Heavy oil fraction (with a high thermal reactivity) is heated to a lower temperature for cracking in the liquid phase. The heated feed streams are combined in a soak drum to provide sufficient time for the completion of the cracking reactions. After the soaker, the products are sent to a flash separator to separate the heavy end as a side product (fuel oil) and the lighter products are sent to the fractionator for separation into LGO, gasoline and the gaseous products. Separating the feed into two fractions will avoid heating the reactive longer alkane chains to high temperatures to keep coke formation and gas production under control to maximize the LGO yield. In naphtha cracking for ethylene production, all reactions are carried out in vapor phase at low pressures to promote β scission reactions for high ethylene yields. Coking of reactor tubes creates a major maintenance problem in naphtha cracking for ethylene production.