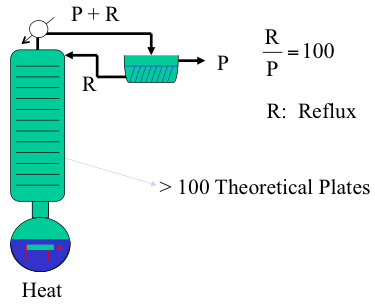
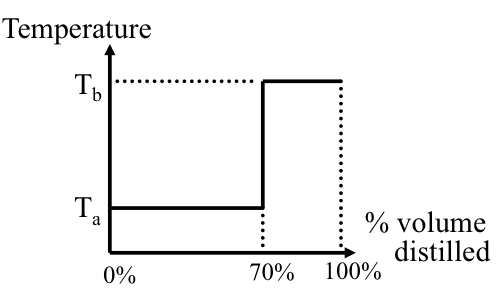
True Boiling Point Distillation (TBP)
This method, described in Figure 4.7a and b, uses a batch distillation operation that incorporates more than 100 theoretical plates and a high reflux ratio (R/P) of 100, as described in Figure 4.7a. This is an idealized method to achieve the best possible separation in distillation, made possible by a large number of theoretical plates (stages) for liquid vapor contact in the column and an extremely high reflux ratio. As an example, consider distillation of a binary mixture of compounds A (70% by volume) and B (30% by volume), with boiling points Ta and Tb, respectively. Figure 4.7b illustrates the distillation curve that would be obtained if this mixture were distilled using the TBP method, with perfect separation of A and B as pure compounds. Because of the TBP distillation conditions, first the lower boiling component A is distilled off without any contamination with B, and following the complete vaporization of A, B is distilled off as a pure compound. Note that because a large number of plates and a high reflux ratio in the column, temperature remains constant during evaporation of A until all of this compound is boiled off, as would be seen in the distillation of a pure compound.