Module 8: Pests and Integrated Pest Management
Module 8: Pests and Integrated Pest Management
Introduction
Agroecosystems have many beneficial species that play important roles in processes such as nutrient cycling, pollination, and pest suppression; but some species, typically called pests, reduce crop or livestock yields and/or quality. This module introduces three types of agricultural pests (insects, weeds, and pathogens) and some of the scientific research, technologies, and management approaches developed to reduce agricultural pest damage.
Goals and Learning Objectives
Goals and Learning Objectives
Goals
- Learn some benefits of insects, some characteristics of insect and weed pests, some challenges associated with insect and weed pest control, and how trophic interactions can contribute to insect pest control
- Learn what IPM is and how to apply the economic threshold concept to interpret if a pest population has reached an economic threshold
- Learn some transgenic pest management technologies and their impact
- Understand how few pest control tactics can select for pest resistance while integrated pest and weed management can contribute to long-term successful weed and pest management
Learning Objectives
After completing this module, students will be able to:
- Describe characteristics of insect pests and factors that make them successful pests, as well as beneficial characteristics of insects.
- Explain some history of agricultural pesticides.
- Describe factors that contribute to pests evolving resistance to pest control strategies.
- Discuss what IPM is and why it is effective.
- Interpret how to apply pest scouting data and distinguish if pests have reached an economic threshold.
- Analyze pest management scenarios and describe the agroecosystem benefits of IPM.
- Describe and compare the characteristics of natural ecosystems and agroecosystems, and explain how trophic level interactions and biodiversity may contribute to pest control.
- Describe characteristics of weed pests and factors that make them successful pests.
- Describe categories of weed management tactics with examples of weed control practices.
- Explain what organisms and factors contribute to crop diseases.
- Explain some recent transgenic pest management technologies and analyze and interpret scientific data about transgenic technologies.
- Differentiate pest control approaches that are likely to be effective in the long term based on IPM principles, and generate or formulate IPM approaches to enhance pest control.
Assignments
Module 8 Assignments Roadmap
Detailed instructions for completing the Summative Assessment will be provided in each module.
| Action | Assignment | Location |
|---|---|---|
| To Read |
|
|
| To Do |
|
|
Questions?
If you prefer to use email:
If you have any questions, please send them through Canvas e-mail. We will check daily to respond. If your question is one that is relevant to the entire class, we may respond to the entire class rather than individually.
If you prefer to use the discussion forums:
If you have any questions, please post them to the discussion forum in Canvas. We will check that discussion forum daily to respond. While you are there, feel free to post your own responses if you, too, are able to help out a classmate.
Module 8.1: Insects and Integrated Pest Management
Module 8.1: Insects and Integrated Pest Management
Ecosystems have many trophic levels of organisms including primary producers, herbivores, omnivores, carnivores; parasites, and decomposers. Agroecosystems are ecosystems managed for food and fiber production that have less diversity and typically fewer trophic interactions than natural ecosystems. But diverse organisms and their trophic interactions provide important functions in agroecosystems including, for instance, decomposition and nutrient cycling; plant pollination, and pest suppression. Organisms that reduce agricultural productivity and quality and are referred to as agricultural pests; these include weed pathogens, insects, and other herbivorous organisms. Mammals that graze or browse crops (ex. deer and rodents), and other arthropod species such as mites and slugs (mollusks), can also reduce crop yields through grazing and seed predation.
Natural Ecosystem and Agroecosystem Comparison
Natural Ecosystem and Agroecosystem Comparison
Pest species can be present in agroecosystems, but not cause significant crop yield loss or livestock productivity reductions. Why? What factors prevent pest populations from reducing yield? One explanation may be that the crop or livestock is resistant to the pest. For instance, a crop plant may produce compounds that fend off pathogen infection or deter insect feeding. And if environmental conditions and resources are ideal, the plant may be able to grow and recover from pest infestation. What other ecological processes and factors might contribute to agricultural resilience to pests or other stresses such as climate change?
Activate Your Learning
Question 1 - Short Answer
Draw a food web pyramid and label the trophic levels as categories of organisms with i. primary producers at the bottom, ii. herbivores next, ii. omnivores and carnivores at the top of the pyramid. Chose a natural ecosystem and list all of the species you can think of that are found at each trophic level in the natural ecosystem. Then draw a second food web pyramid for a type of farm that you are familiar with, and list all of the species you might find at each trophic level. Describe how the natural ecosystem and the agroecosystem compare. How do they differ?
Click for the answer.
You should have many more species at each trophic level in the natural ecosystem. Additionally, the genetic diversity within species in the natural ecosystem is typically greater than in the agroecosystem.
Question 2 - Short Answer
Odum (1997), an Ecologist summarized some of the major functional differences between natural and agroecosystems that are shown in the table below. Consider how your natural and agroecosystem food pyramids offer examples of the below ecosystem differences. How many predatory and parasitic species are there in the natural ecosystem and agroecosystem? How might the presence of predatory and parasitic organisms impact agricultural pests? How might genetic diversity contribute to pest management and ecosystem stability?
Click for the answer.
Although you may not be familiar with parasitic species such as wasps and nematodes, you likely can think of many predatory species: humans, large and small mammals, predatory birds, rodents, fish, and arthropods (ex. beetles, spiders, ants, etc.)
In natural ecosystems there tend to be more niches and a higher diversity of species compared to most managed agroecosystems that are simpler, have fewer predatory and parasitic species, and less genetic diversity within a species. As the table below indicates with fewer trophic interactions, there are fewer species to reduce pest populations and prevent them from reducing agricultural yield and quality. Further, with low genetic diversity within agricultural species and across the landscape, the agricultural system is more vulnerable to pest outbreaks than natural ecosystems.
| Property | Natural Ecosystem | Agroecosystems |
|---|---|---|
| Human Control | Low | High |
| Net Productivity | Medium | High |
| Species and Genetic Diversity | High | Low |
| Trophic Interactions | Complex | Simple, Linear |
| Habitat Heterogeneity | Complex | Simple |
| Nutrient Cycles | Closed | Open |
| Stability (resilience) | High | Low |
Insects
Insects
Insects are the most diverse group of animals that are found in most environments. In the Animal kingdom, Insects are in the Phylum Arthropoda; Arthropods have an exoskeleton of chitin that they shed as they grow; they also have segmented bodies and jointed appendages. In addition to the Class Insecta, the Arthropoda also includes the arachnids (spiders and mites), myriapods (ex. centipedes), and crustaceans (crabs, lobsters, etc.). Insects are distinguished from the other Arthropod classes by the following features:
-
As adults and in some species in the juvenile stages, insects have three body parts: the head, thorax, and abdomen. Although in some insect species, some of the three body parts are fused together and may be difficult to distinguish. See this website for images and more discussion of insect anatomy: Purdue University, College of Agriculture, Department of Entomology, 4-H and Youth: Insect Anatomy [8]
- The adults have antennae on their heads that they use to sense their environment, and they have three pairs of legs or six legs.
 Figure 8.1.1: Honeybees are important pollinator insects. Note the three body parts (head, thorax, and abdomen) two antennae, and three pairs of legs.Credit: MaryAnn Frazier, Department of Entomology, Penn State University [9]
Figure 8.1.1: Honeybees are important pollinator insects. Note the three body parts (head, thorax, and abdomen) two antennae, and three pairs of legs.Credit: MaryAnn Frazier, Department of Entomology, Penn State University [9] - Most insects undergo a morphological change that occurs between the time they hatch from eggs and develop into adults. The morphological change is called either complete metamorphosis or incomplete metamorphosis referring to how significantly the insect's appearance changes from the early stage of development to the adult stage. Go to these links to see images of the types of metamorphosis and read more about insect metamorphosis: ASU School of Life Sciences: Metamorphosis [10]
Check Your Understanding
Question - Multiple Select
Browse the following websites for two major agricultural crop pests. What kind of organisms are they? In what stage of their lifecycle do they cause the most damage to the crop plants?
Click for answer.
Both are insects that undergo complete metamorphosis. They do the most crop feeding and damage in the larval stages when they resemble worms, that are sometimes also called caterpillars. Thus, their common names include the name: worm.
Feeding Types
Insects may be herbivores or omnivores. Herbivorous insects may eat plants by directly feeding on plant tissues such as leaves or roots. Herbivorous insects include caterpillars, beetles, grasshoppers, and ants. Some insects pierce plants and suck plant nutrients from the plant vascular system, typically the phloem, (the cells that transport plant carbohydrates and amino acids); although some insects feed on the xylem, the vascular cells that transport water and nutrients. Examples of piercing-sucking insects include aphids and mosquitoes. By contrast, butterflies and moths have siphoning mouthparts for drinking nectar. Omnivore insects consume multiple kinds of food including other insect prey and plant tissues such as leaves and/or nectar and pollen.
Beneficial insects
Although insect pests are major agronomic pests, only about 1% of insect species are agricultural pests. Insects also contribute to important ecosystem processes, including: i. pollination, ii. predation and parasitism (ex. lady beetles, lacewings, praying mantis, parasitic wasps); iii. decomposition of organic materials such as crop residues and manure (Ex. dung beetles) iv. providing food for other organisms, such as fish and birds. Review the photos below for some categories of beneficial insects, and some of their characteristics here: National Pesticide Information Center [13]



Activate Your Learning
Read the following website: Omnivorous Insects: Evolution and Ecology in Natural Agriculture Ecosystems [16].
Then answer the following questions:
What did scientists observe happened to cotton plants and insect herbivores after cotton plants were injured by herbivorous insects?
Click for answer.
To conserve or maintain predatory insects, what is required? What can farmers do to attract and conserve predatory insects?
Click for answer.
Pest Management
Pest Management
Humans have developed methods of insect and pest control for centuries.
Reading
Read the following brief history of pesticides and then answer the questions that follow:
Pesticide Development: A Brief Look at the History [17]. Taylor, R. L., A. G. Holley and M. Kirk. March 2007. Southern Regional Extension Forestry. A Regional Peer-Reviewed Publication SREF-FM-010 (Also published as Texas A & M Publication 805-124)
Check Your Understanding - Pesticide Development: Brief History
What chemicals were used to control pests from 1700 to the early 1900s?
Click for answer.
When was DDT invented and what was it first used for?
Click for the answer.
When and why was DDT banned?
Click for the answer.
Pesticide Resistance
Soon after the development of DDT in 1939 and the dawn of the modern insecticide era in the 1940s, scientists began to understand that pesticides were not the silver bullet of pest control. Particularly when a pesticide or one effective pest control strategy is relied on, the control tactic acts as a strong selective force for the development of resistance to the tactic in the target pest population. With the continuous application of the same pesticide, individuals that are susceptible to the pesticide are killed, leaving the few resistant individuals that survive to reproduce a offspring that are resistant to the pesticide. See the figure below for an illustration of how frequent reliance on one insecticide can select for a resistant insect population. Further, since many early pesticides were broad spectrum pesticides, the natural enemies of agricultural pest populations were also destroyed, contributing to pest population outbreaks.
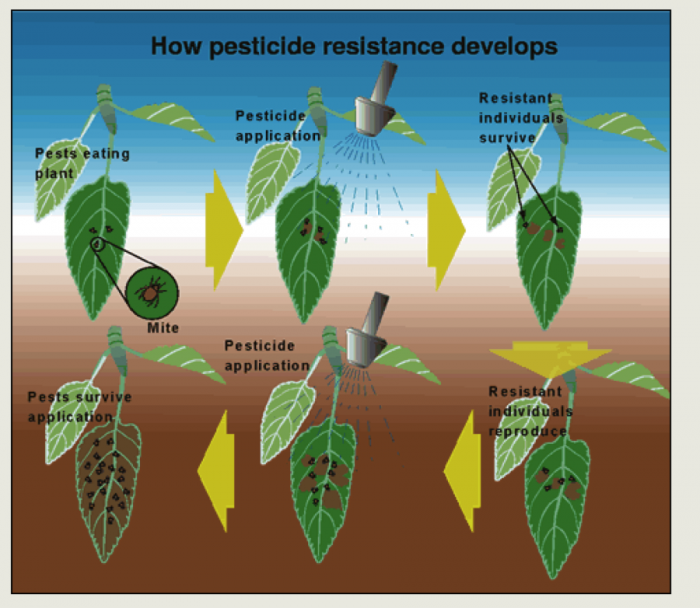
In 1984, the US Board of Agriculture of the National Academy of Sciences organized a committee to explore the science of pest resistance and strategies to address the challenge. A report called "Pesticide Resistance: Strategies and Tactics for Management" was co-authored by the Committee on Strategies for the Management of Pesticide Resistant Pest Populations and published in 1986 by the National Academies Press, Washington D.C. In Chapter 1, G. P. Georghiou (1986) documented the development of pest resistance across multiple pest organisms (see pages 17 and 28 for figure 2 [19] and figure 8 [20]), as well as how difficult and costly it was becoming to develop cost-effective pesticides (see figures 12 and 13 [21] on page 36).
In the report, the Committee recommended using Integrated Pest Management or IPM to reduce the evolution of pesticide resistance and provide more long-term, effective pest control. As early as 1959, a team of scientists (Stern et al.) in California had also proposed that pest control that integrated both biological and chemical control approaches, was needed to prevent pest resistance to pesticides and pest control. Stern et al. (1959) defined terms and concepts that are fundamental to IPM today.
Understanding Economic Thresholds
Understanding Economic Thresholds
Read the following two fact sheets for a description of Integrated Pest Management and the terms that Stern and his colleagues defined in 1959, which are still used today (economic injury level, economic threshold, and general equilibrium position). Then watch the following short video and answer the questions below:
-
The Integrated Pest Management (IPM) Concept [3]. D. G. Alston. July 2011. IPM 014-11. Utah State University Extension and Utah Plant Pest Diagnostic Laboratory
- IPM Pest Management Decision-Making: The Economic-Injury Level Concept [4]. D. G. Alston. July 2011. IPM 016-11. Utah State University Extension and Utah Plant Pest Diagnostic Laboratory
Activate Your Learning: IPM Concept and Decision-Making
Describe three things that are integrated into IPM.
Click for the answer.
On the IPM figure below, which IPM pest population terms from the article could describe the lines labeled A, B, and C?
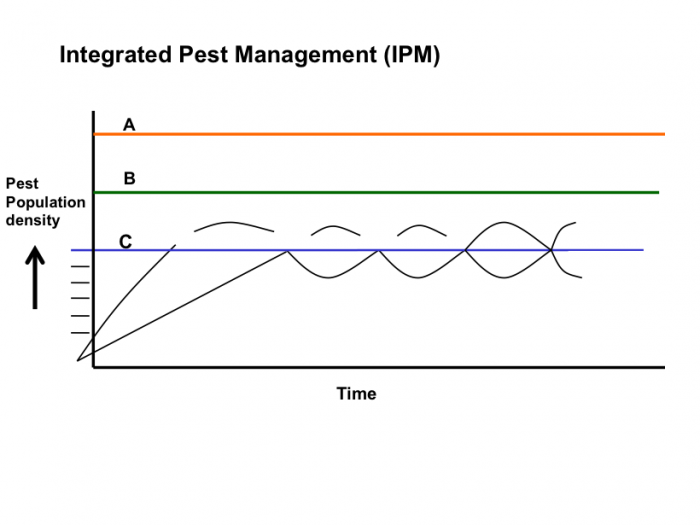
Click for the answer.
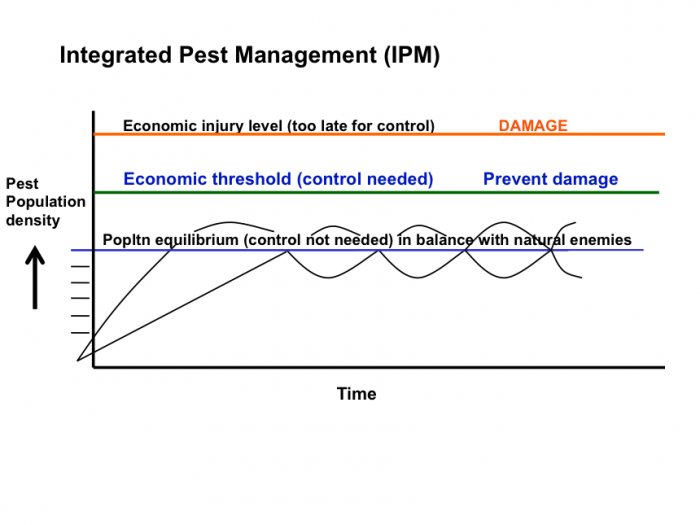
How would you describe the damage that the pest had caused to the crop at each of these pest population densities?
Click for the answer.
Watch the first 4.11 minutes of the below video: Integrated Pest Management (IPM) in Apple Orchards, which describes European Red Mite pests and predatory mites in Pennsylvania apple orchards.
Video: Integrated Pest Management (IPM) in Apple Orchards [22] (8.34)
What are the potential benefits of scouting for the European red mites and predatory mites in Pennsylvania orchards?
Click for the answer.
Formative Assessment: Australian Grain Crop IPM and Determining the Economic Threshold of Potato Leafhoppers in Alfalfa
Formative Assessment: Australian Grain Crop IPM and Determining the Economic Threshold of Potato Leafhoppers in Alfalfa
Part 1: Australian grain crop IPM
Watch the following video that explains IPM adoption in grain crops in Australia; then answer this question:
1. Identify and explain three benefits of utilizing IPM discussed in the Australian video from the GRDC.
Video 1: GCTV2: Integrated Pest Management (5:46)
Narrator: Now another aspect of the overall push for improved farming practices, is how we control pests; and Jane Drinkwater reports on the latest approach to pest control while looking after the environment.
Jane Drinkwater: Australia's crop production systems are forever improving. A prime example is how we manage insect pests. Where once broad-spectrum, often highly toxic, insecticides were used to blanket eradicate insects, there's a move towards a more holistic approach, and with good reason. Integrated pest management, or IPM, presents a win-win, less damage to the environment and to your hip pocket.
Rowan Peel (Mount Pollock VIC: I love the environment and I want to look after the environment, but I have to make a living. IPM has given us the opportunity to do all of these things, both look after the environment and to make more money.
Jane: IPM uses multiple strategies to manage insect pests. One of the tactics is to let an army of the insects’ natural predators, or beneficials, fight the battle for you, and that means holding off on the use of broad-spectrum chemicals.
Rowan: I've probably learned that nature has its way of handling things its own way. You just have to give those beneficials that time. And when you understand that when you are using a broad-spectrum insecticide that you might control it straight away, but you'll get another flight straight in. But you've killed all your beneficials, and you've killed beneficials for other pests later on. And some of these beneficials don't have the lifecycle of an aphid. You know, their lifecycle might be only once or twice a year. And so you know, economically, if you look at the long-term, you're a long way worse off.
Jane: For insects without natural predators, or where the ratio of pests to beneficials is high enough to affect yield, strategies include the application of pesticides to problem areas only and the use of chemicals which target the problem pests, without damaging the beneficial insects. Rowan: We actually treat the seed for earwig infestation to give ita protection. But if there is a further problem, and that may well only be in certain areas of the paddock, which we tend to know where they will be, we will make up a brew of wheat, a little lawsben, and a little bit of vegetable oil. And we'll go out and spread just on that area. So as the earwigs are attracted to that bait, rather than all the other insects.
Jane: Peter Enkelmann’s been using IBM for more than a decade. While his beneficials successfully control silver leaf whitefly, there are still a few pests without natural predators.
Peter Enkelmann (“Riverview” Byee QLD): The chemistry that we use here, it takes out the beneficial insects. So the attitude is to delay spraying any product at all basically, apart from very few natural viruses, right through until the very last.
Jane: And using IPM means, when you do need to pull out the big guns, they're more likely to work.
Peter: One of the big advantages is that resistance to our traditional chemistry is just dropped dramatically.
Jane: But how do growers know when to take action? Well thanks to research funded by GRDC, entomologists have data on the density of pests in each crop that will lead to economic damage. Growers measure the number of pests in their fields and only take action once they've reached this threshold.
Hugh Brier (Senior Entomologist, Primary Industries and Fisheries, DEEDI QLD): So the short-term gain is you might avoid unnecessary sprays. Another short-term gain is by not spraying when you didn't need to, you might avoid flaring another pest which is more expensive to control, so that's another benefit. Longer term, if you avoid spraying unnecessarily, you build up beneficials in the whole system and the system is much more stable.
Jane: Fundamental to successful integrated pest management is the ability to correctly identify pests and beneficial species, and to regularly monitor both populations.
Hugh: In row crops, we use a bed sheet. So we'll go and we shake the plants from meter of row and that shakes all the insects out, or a lot of them out onto the bed sheet and you can count them.
Jane: With IPM leading to lower costs and better environmental outcomes, GRDC views it as an important step forward. Apart from funding IPM Research, GRDC also provides information and training for growers.
David Shannon (GRDC Southern Region Panel Chairman): We have run a series of workshops, IPM workshops. We also work with the grower groups so that grower groups can scale up their grower members on the use of IPM.
Jane: And it's well worth getting up to speed.
Rowan: I find the system of IPM very easy because it's not an almost do nothing, but you just don't worry about it anywhere near as much.
Jane: With IBM's effectiveness in controlling insects, while reducing costs both financial and environmental, it's here to stay.
Rowan: IPM for us has cut down our chemical usage, insecticide usage a long way and you feel better for not using it.
If the video does not load for you, go to GCTV2: Integrated Pest Management [23]
Part 2: Determining the Economic Threshold of Potato Leafhoppers in Alfalfa
Read the Penn State University Potato Leafhopper on Alfalfa Fact Sheet [24].
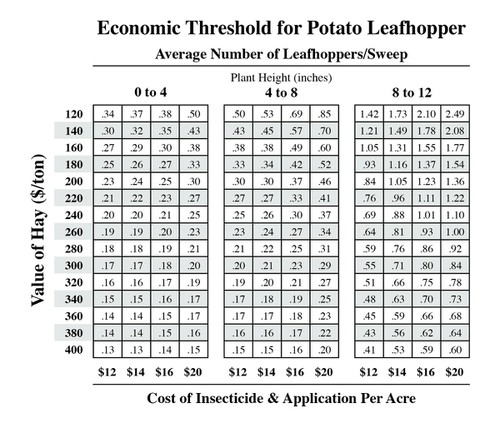
Scenario
Assume that you followed the procedure described in the Penn State fact sheet to scout for Potato Leafhoppers in an alfalfa field by sweeping 20 times with your sweepnet in each of 5 different locations in the alfalfa field. The number of leafhoppers that you found in the 5 different locations was: 15, 12, 16, 7, 13 when the alfalfa crop was about 11 inches tall. You would like the alfalfa to grow about 25-30 inches height before harvesting it for hay, this could require 2 to 3 more weeks of growth, depending on rainfall. Based on current alfalfa hay prices in your region, you estimate your alfalfa hay is worth about $250/Ton, and the insecticide you would spray to control the leafhoppers would cost about $16/A. If you spray the alfalfa field, it cannot be harvest until 7 days after spraying the insecticide; and due to toxicity to bees, the alfalfa should not be sprayed if it is flowering.
Answer the following questions:
- Calculate the average number of leafhoppers per sweep. Add the number of potato leafhoppers from the 20 sweeps in each of the 5 locations (20 X 5= 100 sweeps). Divide by 100 to calculate the number of leafhoppers per sweep. Use the Economic Threshold Table from the Fact Sheet for Potato Leafhoppers, shown here. Has the insect population reached an economic threshold for your crop at this height?
- Based on the average number of leafhoppers per sweep, what should you do? Why?
- If your crop height was 7 inches tall and you had the same number of leafhoppers per sweep that you calculated here, would your pest management decision change and how?
- Discuss at least two potential benefits of using the economic threshold decision tool rather than spraying as soon as potato leafhoppers were first visible.
Files to Download
Module 8 Formative Assessment Worksheet [25]
Submitting Your Assignment
Please complete the Module 8 Formative Assessment in Canvas.
Module 8.2: Weeds, Transgenic Crops for Pest Management, and Pathogens
Module 8.2: Weeds, Transgenic Crops for Pest Management, and Pathogens
Weeds are a major crop pest that persist in agricultural ecosystems, and significant resources are allocated to studying weeds and developing technologies to control them. What characteristics make weeds such significant pests and how can they be controlled? We will employ the plant lifecycle terms that you learned about in Module 6 to describe weed lifecycles and identify effective weed control practices. We will also explore how the principles of integrated pest management are applied in weed management; and you will learn about transgenic pest control practices that have been widely adopted for insect and weed control; as well as some plant pathogen management principles.
Weeds
Weeds
A weed is a plant that is not wanted or a plant growing in the wrong place. In agricultural systems, weeds tend to be unwanted because they compete with crops for light, water, and/or nutrients, and can reduce crop yield and/or quality, particularly if weeds are permitted to grow and reproduce. Weeds may reduce crop quality through contamination with seeds or plant parts that may be toxic, or of poor nutritional or culinary quality (produce off-flavor compounds). Some weeds may harbor crop insect pests or pathogens; and when weeds have a significant negative impact, they can reduce the economic value of agricultural land. On the other hand, if weeds are not numerous enough to reduce crop yield and quality, weeds can provide some agroecosystem benefits. For instance, weeds can provide:
-
protection from soil erosion
- pollen, nectar, and habitat for beneficial organisms and wildlife
- forage for grazing animals
Competitive Characteristics of Weeds
Weeds tend to be plants that are adaptive and competitive in a range of environmental conditions. They typically have seeds or perennial storage organs that enable them to grow rapidly and produce aboveground canopies that compete with crop plants for light, and root systems that compete for nutrients and water. Annual weed species often grow and mature relatively quickly, producing seeds earlier than crops. To increase survival of their offspring, annual weeds often produce many seeds, and some species produce large seeds. Strategies to control annual weed species target terminating them early, to prevent them from competing with crops and producing seeds.


If perennial weeds are growing from the small seeds they produce, they establish more slowly than annual crop seeds. But seeds are not their primary form of reproduction, recall that perennial plants often spread and reproduce via established storage organs such as taproots, tubers, bulbs, and rhizomes (belowground modified stems that store reserves and enable a plant to spread horizontally), or aboveground stolons or storage stem bases. Perennial weeds growing from storage organs can be very competitive with crop plants. If perennial weed storage organs are cut and distributed over a larger area and reburied or partially covered, they can also establish and spread across a larger area. If weed storage organs are left on the soil surface to freeze and thaw over winter or desiccate in mid-summer, then tillage can terminate perennial storage organs. Repeated mowing of plant regrowth may weaken or deplete plant reserves, particularly if it is prior to the end of the growing season when perennials tend to translocate plant reserves to storage organs. Chemical control of perennials is also often most successful at this time when herbicides can be translocated to storage organs.



Weed Survival Characteristics
Weed Survival Characteristics
In addition, many weeds have traits that enhance their survival and reproductive success such as: i. hard-seeds or seeds that can remain dormant for long time periods until environmental conditions for germination are good, enhancing weed seed success, ii. plant protective characteristics such as thorns, toxic tissues, protected growing buds, iii. adaptive growth to a wide range of environmental conditions also referred to as plasticity. For example, in a field or lawn that is grazed or mowed to a short height to control weeds, adaptive weeds can produce leaves very close to the soil surface and flowers on short stems below the mowing height.


Check Your Understanding
Read about the Velvetleaf weed species (Abutilon theophrasti L.) at Velvetleaf. [29]
Velvetleaf has hard seed. How long can the seed survive? On the website click on the link that discusses Velvetleaf Adaptation and Stress. What examples does the author use to illustrate velvetleaf plasticity or ability to adapt to its growing conditions?
Click for the answer.
Activate Your Learning: Weed Control Practices
Recall what you have learned about crop plant lifecycle classification and characteristics in Module 6.
Read the Australian Department of the Environment website that describes Integrated Weed Management [30]. Click on and read the links that describe each type of weed management technique. After you have read both of the above readings, answer the questions below.
Question 1 - Essay
Review examples of the four weed control strategies discussed in these two online publications. Explain at least two specific weed control strategies that are likely to be effective for controlling annual weeds and explain why they are effective for annual weeds.
Click for the answer.
Annual weeds typically germinate, grow and develop to maturity and seed production quickly, and therefore can offer significant competition with crop plants for light, nutrients, and water. They can also produce seeds and increase weed population pressure relatively quickly. Therefore, annual weeds should be terminated early and especially prior to they produce seeds. Because annual weed species do not allocate significant resources to below ground storage organs, they can be terminated with mechanical or physical control tactics such as: plowing, cultivation, hoeing, removal by hand, hay making, mowing or grazing, soil mulching, and flaming. Allowing weed seeds to germinate and then terminating them with light tillage (stale seedbed) strategically uses tillage for weed control. Annual weed germination and establishment can be suppressed with cultural control practices such as crop rotation, rotating crops with different seasonal life cycles, successive planting (double or triple-cropping); the integration of cover crops; and managing for competitive crops with early crop planting and good crop management practices (competitive crop varieties, soil fertility and health management).
Chemical control with herbicides applied at the recommended time, and rotating or varying herbicide chemistry can reduce the evolution of herbicide-resistant weeds. Biological control can also reduce weed populations and may include conserving habitat for weed seed predators such beetles, small rodents, and birds by integrating cover and perennial crops on a farm, and avoiding pesticides that can reduce weed seed predatory populations. In addition, in some cases, browsing or grazing animals or specific pathogens of weeds such as bacteria or fungi are sometimes introduced.
Question 2 - Essay
Describe at least two weed control strategies that are likely to be effective to control perennial weeds. Explain why.
Click for the answer.
Perennial weeds have below-ground storage organs that they can regrow from (ex. tubers, rhizomes, stolons, and bulbs); therefore mechanical control strategies such as tillage, cultivation, and hoeing can break up and distribute perennial weed storage organs, facilitating the spread of perennial weeds. Mechanical weed control tactics can be effective if they can bring the majority of the storage organ to the soil surface to desiccate or freeze, thaw, desiccate and die. Perennials typically begin storing reserves for spring regrowth in late summer and early autumn. Therefore, frequent mowing or flaming can deplete a perennial weed’s storage reserves, if the tactic is repeated multiple times, particularly during summer before the plant begins replenishing storage reserves.
Applying systemic herbicides that are taken up by the plant and translocated to storage organs is also most effective in late summer and early fall when perennial plants are replenishing storage reserves. Rotating or varying herbicide chemistry can also reduce the evolution of herbicide-resistant weeds.
Cultural control strategies for perennial weed control include crop rotation between perennials and annual crops, planting perennial crops with competitive annual companion crops that are harvested early, and managing for competitive crops with early crop planting and good crop management practices.
Biological control practices as described for annual weeds can reduce perennial weed populations, and be particularly cost-effective in rangelands where other practices are often cost-prohibitive. Biological control strategies may include introducing browsing animals such as goats that will eat thorny perennial weeds, or application of weed-specific pathogens such as bacteria or fungal spores.
Herbicide Resistance
Herbicide Resistance
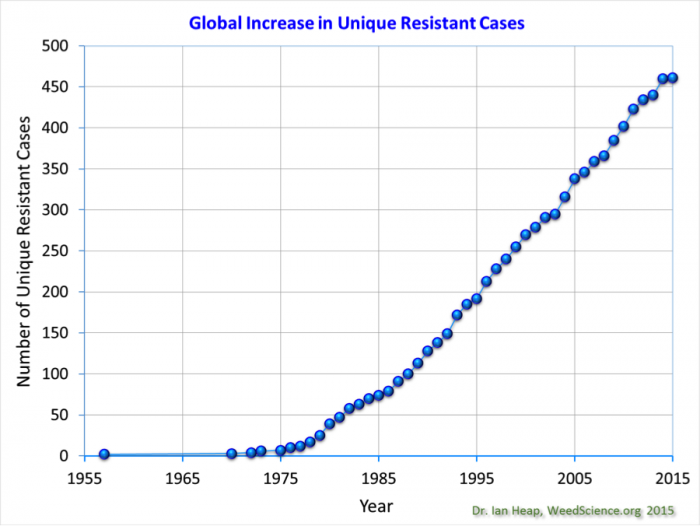
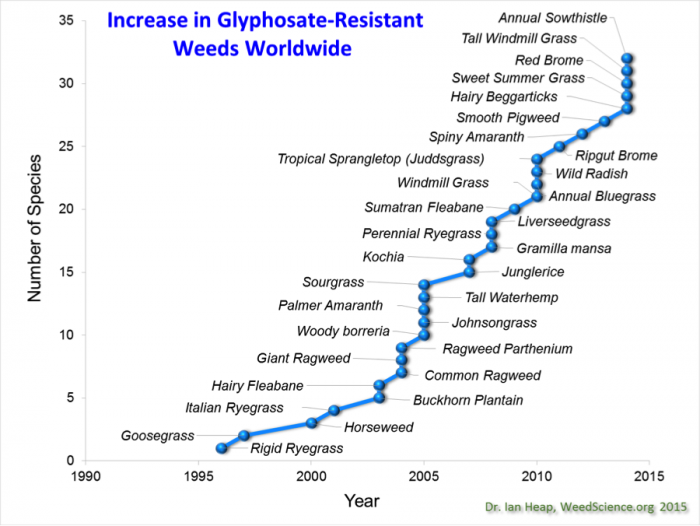
Although integrated pest management was introduced in the 1980s, the number of weeds that have evolved resistance to new herbicides continues to grow (See Figure 8.2.8 below).
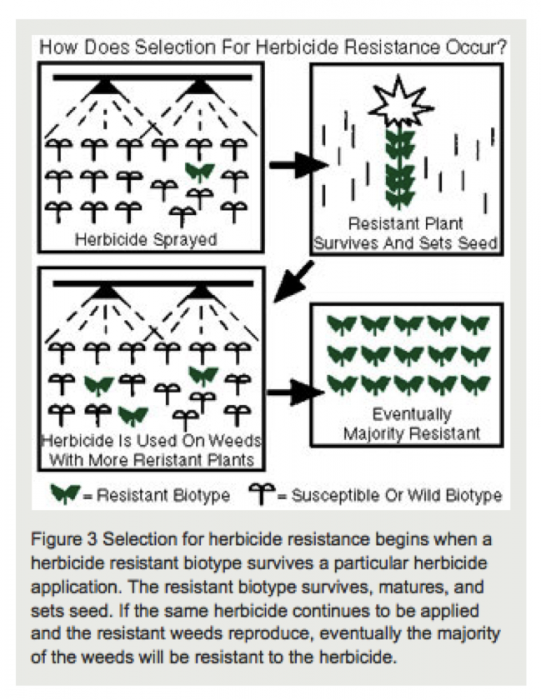
Similar to other pests, weeds evolve resistance when exposed to the same strong selective force, such as an application of the same herbicide over consecutive years. When the same herbicide is applied numerous times to a field, susceptible weeds are killed, leaving resistant individuals to reproduce and dominate the population, as illustrated in figure 8.2.9 below.
Integrated Weed Management
Integrated weed management (IWM) is an IPM approach for weeds that can provide long-term weed control of weeds by integrating multiple control strategies. Some weed scientists have described IWM as utilizing “many little hammers” as opposed to continuously employing one “big hammer” such as an herbicide (Liebman & Gallandt, 1997). Weed control tactics fall into the IPM control categories that you learned about for insect control in Module 8.1. Examples of weed control practices include the following:
-
Cultural control practices are management practices humans can employ to prevent weed establishment and maintain vigorous crop growth. Examples include: crop rotation with crops of different life cycles and seeding densities, planting certified seed that is managed to have minimal weed contamination, planting adapted crop varieties, adjusting row spacing, population density, and timing for a competitive crop and successful crop establishment, maintaining soil health and fertility, and using practices that prevent weed establishment such as cover crops and mulching.
 Figure 8.2.10: Straw mulch to suppress weeds in garlic.Credit: Heather Karsten
Figure 8.2.10: Straw mulch to suppress weeds in garlic.Credit: Heather Karsten Figure 8.2.11: Buckwheat (Fagopyrum esculentum L. ) cover crop planted mid-season to smother weeds, produce organic matter and provide habitat for beneficial insects.Credit: Anna Santini
Figure 8.2.11: Buckwheat (Fagopyrum esculentum L. ) cover crop planted mid-season to smother weeds, produce organic matter and provide habitat for beneficial insects.Credit: Anna Santini

 Figure 8.2.12: Crop rotation of summer annual row crops such as corn (first photo) with densely seeded perennial alfalfa (second photo), and/or densely planted winter annual wheat and spring oats (third photo, wheat is on the right, oats are on the left).Credit: Heather Karsten
Figure 8.2.12: Crop rotation of summer annual row crops such as corn (first photo) with densely seeded perennial alfalfa (second photo), and/or densely planted winter annual wheat and spring oats (third photo, wheat is on the right, oats are on the left).Credit: Heather Karsten - Mechanical or physical weed control includes practices such as plowing, cultivation, hoeing, targeted hand-weeding, and flaming.
 Figure 8.2.13: This flex tine cultivator is used to control weeds when they are very small.Credit: Heather Karsten
Figure 8.2.13: This flex tine cultivator is used to control weeds when they are very small.Credit: Heather Karsten Figure 8.2.14: Rotary harrow for weed cultivation.Credit: Heather Karsten
Figure 8.2.14: Rotary harrow for weed cultivation.Credit: Heather Karsten - Biological control: conserving or introducing herbivorous insects, grazing or browsing animals, or plant pathogens to reduce weed populations. Biological control of weeds is typically used on extensive rangeland where other more labor intensive or expensive methods are not cost effective.
- Chemical control: the application of herbicides, or the use of herbicide-resistant crops with herbicide applications to control weeds
- Genetic resistance: selecting crop varieties that are well adapted to an environment and competitive with weeds. Herbicide-resistant crops may also be considered a form of genetic resistance.
Transgenic Crops for Pest Control
Transgenic Crops for Pest Control
Transgenic crops or animals are often referred to as GMO’s or genetically modified organisms. This is misleading because all cultivated crop plants and livestock have been genetically modified through centuries of human selection and traditional breeding. A more accurate name for the genetically engineered organisms that are referred to as GMOs, is transgenic organisms. Transgenic crops or livestock contain genetic material that was transferred from a different species through biotechnology techniques or genetic engineering.
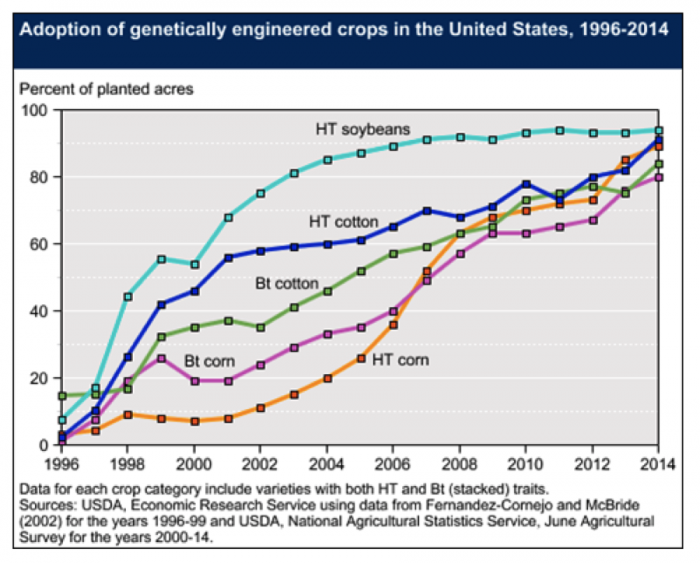
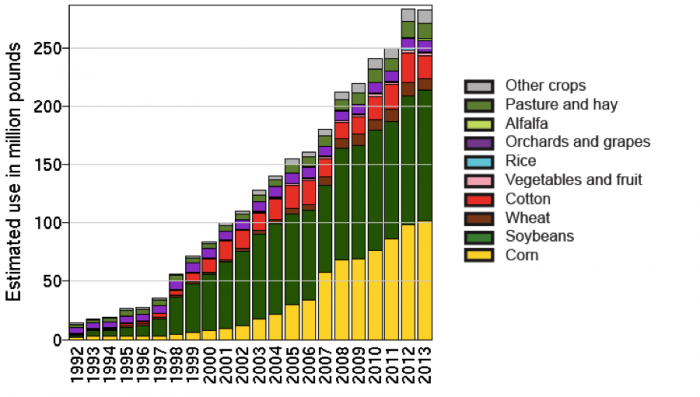
In the 1980s, agricultural input companies began developing and using transgenic techniques to develop new crop varieties. The first traits that were inserted into major crop plants and commercialized on a large scale were genes from two different species of bacteria. The transgenic traits were for insect resistance (Bt) and resistance to the herbicide glyphosate (commercially marketed as Round-up). Since the first commercial release in 1996, these technologies have been widely adopted in the US and other parts of the world (See Figure below).
Insect Resistant Bt Crops
Insect Resistant Bt Crops
Bt is an abbreviation for Bacillus thuringiensis a bacteria that produces an enzyme that is toxic to the digestive system of insects in the Beetle; and Moth and Butterfly families. These two insect families include some major crop pests. Scientists have transferred the genes that code for the production of the toxins into crop plants. Because the Bt trait confers insect pest resistance, the adoption of Bt corn and Bt cotton has contributed to a significant reduction of insecticide use in these crops (See Figures 8.2.16- 8.2.18 below).
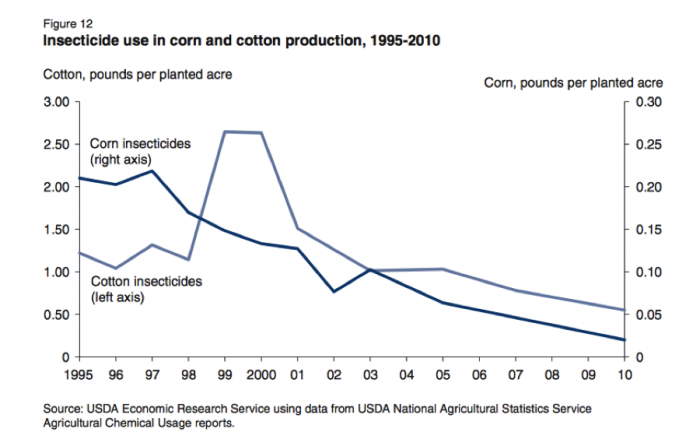
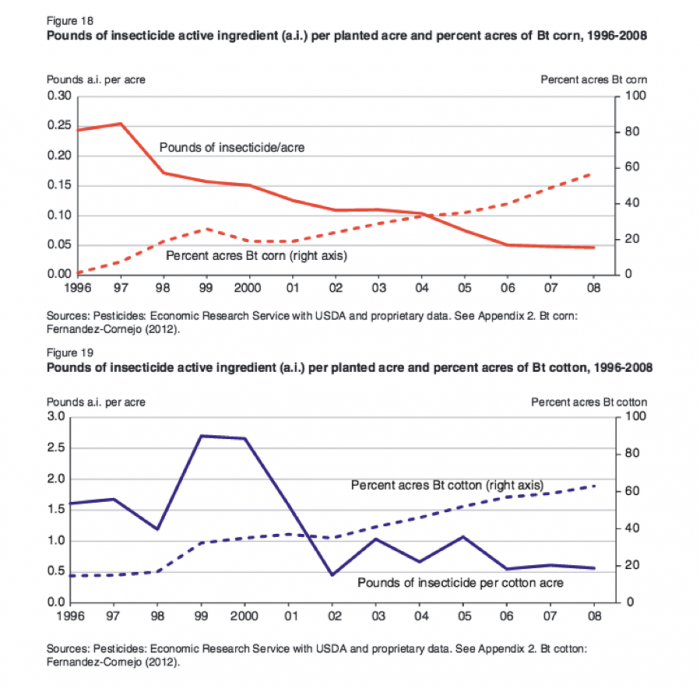
Figure 8.2.18: (Figure 19) Pounds of insecticide active ingredient (a.i.) per planted acre and percent acres of Bt cotton, 1996 - 2008.
Reading
Read this summary of the use and impact of Bt corn, in the following online article “Use and Impact of Bt Maize [2]” by: Richard L. Hellmich (USDA–ARS, Corn Insects and Crop Genetics Research Unit, and Dept of Entomology, Iowa State Univ, IA) & Kristina Allyse Hellmich (Dept. of Biology, Grinnell College, IA). 2012 Nature Education.
Check Your Understanding
Many Bt corn hybrids marketed today contain Bt Cry proteins that are toxic to the corn rootworm and are “stacked” or “pyramids”. To what does this stacked or pyramid in Bt hybrids refer?
Click for the answer.
Name three benefits of Bt corn for farmers.
Click for the answer.
To prevent the evolution of pest resistance to Bt, what practices are most recommended?
Click for the answer.
Prior to the development of Bt crops, spores of the bacteria Bacillus thuringiensis were sometimes used as biological control for insect pests in forestry and agriculture, often on organic farms. Initially, commercial Bt crops were released in the US without any regulations to prevent resistance. But science had shown that pest populations could quickly evolve resistance to Bt, and planting Bt crops on a large scale across the agricultural landscape would create a strong selective force for pests to evolve resistance to Bt. Therefore, in response to public concern about the high risk of pests evolving resistance to Bt, the EPA convened a committee that developed a resistance management plan for Bt crops.
In addition to the crop expressing a high dose of the Bt toxin, to prevent or delay pest resistance to Bt farmers who plant transgenic Bt corn and cotton, are required to plant a refuge, a percentage of the crop field or a field close by that does not express the Bt trait. The refuge area conserves a population of insects that are susceptible to Bt, so that the susceptible insects can reproduce with insects that might have resistance to Bt, sustaining some Bt-susceptible individuals in the population. Depending on the presence of Bt crops in a region, the EPA regulation requires that farmers plant between 5% and 20% of their crop field without the Bt trait. For stacked Bt corn hybrids (with 2 or more Bt traits) farmers must plant the required refuge for each Bt trait that their crop expresses. For more information on refuge requirements, read Insect Resistance Management and Refuge Requirements for Bt Corn [36], from the University of Wisconsin.
Pest Resistance to Transgenic Bt Crops
Despite the refuge requirement in the US, western corn rootworm resistance to Bt corn was reported in multiple Midwestern states (Jakka et al., 2016). The first reported Bt-resistant corn rootworm populations were found in cornfields in Iowa that had been planted to Bt corn consecutively for at least three years, and the authors suggested that the fields likely did not include refuge corn (Gassman et al., 2011). Additional studies also found that the Bt toxin dose was not sufficiently high to delay the evolution of insect resistance and that corn rootworm could evolve resistance to additional Bt toxins in three to seven generations (Gassman, 2016). Further, in 2013 pest resistance to Bt crops was reported in 5 of 13 major pest species in a survey of 77 studies from eight countries across five continents, where resistance management requirements and enforcement varied. Practices that delayed resistance to Bt included the Bt crop expressing a high dose of the Bt toxin and an abundance of refuge crop planting (Tabashnik, et al., 2013). In accordance with integrated pest management principles, entomologists also recommend that other control tactics be utilized to control pests targeted by Bt crops.
In a number of countries (ex. most countries in the European Union), Bt crops and transgenic crops were not approved for commercial production. Concerns about the potential human health and ecological risks of transgenic crops limited acceptance of Bt crops and other transgenic crops. Applying the precautionary principle, some policy-makers and the public require more research and long-term assessment of transgenic traits on human health and ecosystems. An interest in protecting domestic seed markets and companies may also contribute to policy decisions to prohibit the adoption of transgenic seeds produced by multi-national seed companies.
Herbicide Resistant Crops
Herbicide Resistant Crops
Herbicide-resistant (or tolerant) crops, such as glyphosate-resistant crops are transgenic crops that are resistant to the herbicide glyphosate. Glyphosate is a broad-spectrum herbicide that controls a wide range of plants and breaks down relatively quickly in the environment; it was first marketed under the trade name: Round-up. Round-up Ready soybeans were released in the US in 1996, and since then, additional glyphosate-resistant crops (corn, cotton, canola, sugarbeet, and alfalfa) have been developed and widely adopted in the US and other countries (Fernandez-Cornejo J. and S. J. Wechsler, 2015; Benbrook, 2014; Duke and Powles, 2009). See Figure 8.2.15 on the Transgenic Crops for Pest Control page: Adoption of genetically engineered crops.

Herbicide-resistant (HR) crops such as glyphosate-resistant crops have facilitated the increased adoption of no-till or direct seeding of some HR crops because tillage is not needed for weed control. Once a crop has emerged, the risk of glyphosate herbicide damage to the HR crop is eliminated, making it easier for farmers to plant crops and control weeds without tillage. However, although Bt crops reduced insecticide use, the glyphosate herbicide must be applied to glyphosate-resistant crops to control weeds. Since they were first introduced in 1996, glyphosate use has increased. See the Figure 8.2.20 from the USGS Pesticide National Synthesis project below.
In addition, in contrast to Bt crops, the EPA did not require farmers to employ a glyphosate resistance management plan or refuge, and the number of weeds that are resistant to glyphosate has increased. Weeds have evolved resistance to glyphosate particularly in cases where farmers consistently applied glyphosate to manage weeds in HR crops and terminated cover crops and/or perennials with glyphosate prior to planting an HR crop. See the Figure 8.2.21 from the International Survey of Herbicide Resistant Weeds illustrating the increase in glyphosate-resistant weeds below.
Stacked Herbicide-Resistant Crops
When the number of glyphosate-resistant weeds increased and became difficult to control, the agricultural-input industry developed transgenic herbicide-resistance crops that are resistant to additional herbicides. Dow AgroSciences developed a transgenic trait for resistance to 2,4-D, an herbicide that controls broadleaf weeds (dicot plants) and the company stacked or added the trait to soybean and cotton crops that also have resistance to glyphosate. And Monsanto produced a transgenic trait for resistance to an herbicide called dicamba that they stacked (or added to) soybeans that have glyphosate resistance. Dicamba and 2,4-D herbicides are volatile, and there is a risk that when the herbicides are sprayed, they will drift into neighboring fields and field edges, potentially damaging other crops and other plants. Wild plants in field edges and natural ecosystems often provide habitat for beneficial organisms, such as pollinators, pest predators, and wildlife. In 2017, Monsanto's crops with stacked dicamba and glyphosate resistance were available for use in some midwestern and southern states, where glyphosate-resistant weeds were particularly problematic. In 2017, there were so many reports and complaints from farmers about crop damage due to dicamba drift, that the states of Arkansas and Missouri banned dicamba spraying for some of the growing season. The EPA also investigated the complaints, and in autumn 2017, the EPA announced that the companies had agreed to new steps to reduce the risk dicamba drift with dicamba-resistant crops. For more information, see the EPA Registration of Dicamba for Used on Genetically Engineered Crops. [39]
We will explore concerns about the stacked, herbicide-resistant technologies and tactics to manage glyphosate-resistant weeds more in the Summative Assessment.
Plant Pathogens
Plant Pathogens
Pathogens include fungi, bacteria, nematodes, and viruses, all biological organisms that can cause disease symptoms and significantly reduce the productivity, quality, and even cause the death of plants. Pathogens can also infect agricultural animals, but for this module, we will focus on plant pathogens. Read the following brief overview of plant pathogens, Plant Disease: Pathogens and Cycles [40].
Pathogens can be introduced and spread to host plants in many ways. Bacteria and fungal spores can be transferred by wind, in rain, and from the soil via rain splashing onto plant tissues. Insects can vector or infect a plant with a pathogen when they feed on an infected host plant, and then move and feed on an uninfected plant. Pathogens can also spread through infected seeds, transplants, or contaminated equipment, irrigation water, and humans.


Plant Disease Triangle: Plant pathologists have identified three factors that are needed for a plant disease to develop:
i. a susceptible host Some pathogens have a narrow host plant range, meaning they can infect just a few host species. For instance, the primary host crops of Late blight (Phytophthora infestans) are tomato and potato. For more information see Tomato-Potato Late Blight in the Home Garden [42]. By contrast, pathogens with a wide host plant range can infect many different host species. There are almost 200 plant species that can be infected by Bacterial wilt (Ralstonia solanacearum). For more information, see Bacterial Wilt - Ralstonia solanacearum [43].
ii. a disease-causing organism (pathogen). Plant pathogens include fungi, bacteria, viruses, and nematodes. For examples, again, see the reading: Introduction to Plant Diseases [5], A. D. Timmerman, K.A. Korus. 2014. University of Nebraska-Lincoln. Extension. EC 1273.
iii. a favorable environment for the pathogen. Pathogens usually require specific humidity and temperature conditions for pathogen infection and disease symptoms to manifest. For instance, Late Blight disease symptoms are most likely to occur when the weather is cool and wet.
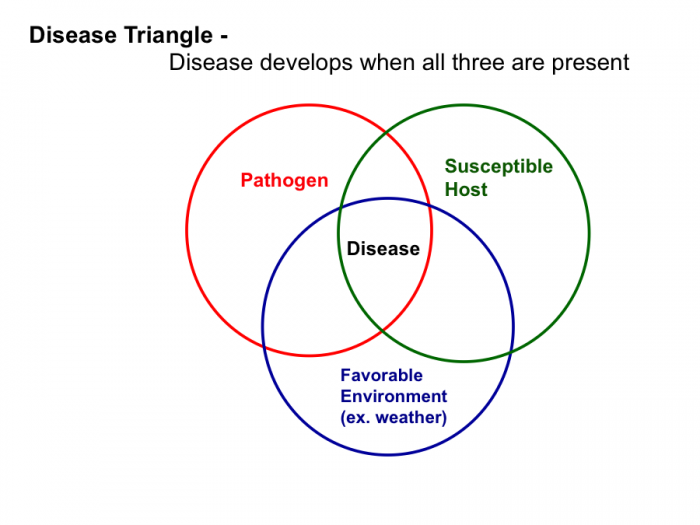
Disease Diagnosis
The three disease triangle factors are important for diagnosing the cause of disease symptoms. Pathologists consider the weather, environmental conditions and the host species to diagnosis what pathogen is causing disease symptoms. Pathologists also consider other factors that could favor and help diagnose a disease, such as i. the field history, particularly what crops and pathogens were present in the past, ii. current crop management practices, iii. when disease symptoms were visible, and on what other species. To assist farmers and others with disease diagnosis, many land-grant universities in the US have crop and animal diagnostic disease clinics where one can submit diseased tissue samples with detailed information that can aid in the diagnosis, such as the host species, environmental conditions, the site history, and management.
Pathogen Management
Although disease control practices could be categorized into the pest control approaches that were discussed earlier for managing insects and weeds (genetic, cultural, chemical, etc.), plant pathologists typically describe pathogen control tactics with more specific language. For instance, Exclusion tactics involve rejecting infected transplants from being introduced to a farm.
Prevention or Avoidance of pathogen introduction and spread tactics include:
- crop rotation, particularly for pathogens with narrow host ranges
- sanitizing equipment for planting, trellising, pruning, and harvesting
- managing for healthy vigorous crops with optimal soil and water nutrient management
- managing to avoid environmental conditions that promote pathogens, such as avoiding very humid conditions due to over-watering, or promoting drying of plant surfaces with wide row spacing to facilitate air flow, or using drip-tape irrigation that waters plants at or below the soil surface versus over the canopy
- using physical barriers such as row covers, mulch to reduce water splashing; and high tunnel/hoop houses or greenhouses to prevent the introduction of rain and wind-borne pathogens



Genetic resistance to pathogens is a very valuable and important pathogen control tool. Many plant breeding programs select for genetic resistance to pathogens. When available, pathogen resistance traits are included in most crop variety descriptions to help growers select appropriate crop varieties for their farm.

If disease symptoms develop, infected plants may be Eradicated or destroyed. And materials that may have been contaminated with pathogens, such as the soil and planting containers, can be heated to very high temperatures with pasteurization equipment or through solarization. For instance, soil may be solarized by placing black plastic over the crop bed (planting zone) during the warm season to increase the soil temperature and destroy pathogens prior to planting the crop.
Therapy or Fungicides (chemical control) may be applied to infected plants to terminate pathogens. Particularly when plant pathogen symptoms are identified early and favorable weather conditions for the pathogen are projected to continue, fungicides can prevent disease spread and significant economic losses. In some high-value crop systems, the soil may be fumigated prior to planting crops.
Similarly, in agricultural livestock systems, animals with disease symptoms can be treated with antibiotics. And in some livestock production systems, antibiotics and vaccinations are administered to animals to prevent diseases and pathogen infection.
Activate Your Learning
Read Nonchemical Disease Control [44], from Colorado State University Extension and identify some pathogen control tactics that could also qualify as other types of pest control categories that we have explored in this module (such as genetic, cultural, and chemical control).
Click for the answer.
- Cultural control strategies: Prevention and Avoidance through crop rotation and management of the crop environment. Exclusion and Eradication of inoculum through sanitation to the survival of plant pathogens on crop residues and agricultural equipment and managing for healthy vigorous crop growth.
- Genetic: Resistance, Selecting and breeding crop varieties for resistance to plant pathogens is one of the primary means of disease management, particularly in agronomic crops.
- Chemical: Fungicides that are toxic to pathogens
- Physical or mechanical: Protection of crops via barriers such as plant netting or soil mulching. Eradication could also be qualified as a physical control strategy
- Regulatory: Quarantines
Summative Assessment: Herbicide-Resistant Weed Interpretation and Management of Multiple Pest Types
Summative Assessment: Herbicide-Resistant Weed Interpretation and Management of Multiple Pest Types
Note
Please read through the entire assignment before you begin the assignment. Once you have done that, return here to follow the link and review the information.
Explore the International Survey of Herbicide Resistant Weeds [38] to examine the trends of herbicide-resistant weeds and answer the questions below. By going to the "US State Map [45]" to click on different states and "map of different countries [46]" to move your cursor over different counties, compare the number of herbicide-resistant weed species across a range of geographical regions.
You will also use the following information in the proposed scenario:
Dow AgroSciences developed a transgenic trait for resistance to 2,4-D, an herbicide that controls broadleaf weeds (dicot plants), that has been transferred to soybean, corn, and cotton crops. The trait is stacked or added to soybeans that also have resistance to glyphosate, and another herbicide called glufosinate.
Monsanto has produced a transgenic trait for resistance to an herbicide called dicamba, that they are stacking (or adding to) soybeans that have glyphosate resistance. Some formulations of the dicamba herbicide are volatile, and there is a risk that when farmers spray dicamba it will drift into neighboring fields and field edges, potentially damaging other crops and wild plants in field edges and natural ecosystems. These field edges and other plants often provide habitat for beneficial organisms, such as pollinators, pest predators, and wildlife.
Please also read these short NPR articles on dicamba:
With ok from EPA use of controversial weedkiller is expected to double [47]
A wayward weed killer divides farm communities harms wildlife [48]
If you are interested in more information about the use of dicamba and Arkansas' recent restrictions on the herbicide you may read or listen to the following brief (3-minute) story: Arkansas defies Monsanto moves to ban rogue weed killer [49]
Directions
Read the following scenario and in approximately 450-500 words answer the questions below. I suggest you write your response in a separate document and then copy and paste it into Canvas. Once you have posted your own answers, you need to respond to ONE classmate. Your response should be approximately 150 words.
Assume you manage a 200-acre corn and soybean farm in Southern Pennsylvania. You keep up with the latest technological advances in farming and use seeds from either Dow or Monsanto depending on what your seed salesperson recommends. You are proud of your farm and strive to keep your crops free from both weeds and harmful insects that could damage your crop and cut into your profits. Your immediate neighbors on the East side have a large organic vegetable production farm. Based on what you have observed on the above website, the information mentioned above, and from what you have learned through the readings in this module, answer the following questions:
- What are some potential problems you might encounter if you adopt seeds with the new herbicide traits listed above? How do these problems differ between now and in the future?
- What other weed-control strategies could you use to control glyphosate-resistant weeds?
- How might your pest management system affect your neighbors?
- Are there strategies you can use to mitigate potential harm to your neighbors’ fields?
- If you were farming in Thailand or Egypt instead of in Pennsylvania, what might explain differences in the number of herbicide-resistant species you encounter there compared to your farm in the United States?
- If you farmed in Canada, Australia, or Western Europe what might explain the herbicide-resistant species you encounter there?
Consider the following possible questions when responding to a classmate:
- How do their answers differ from yours?
- Are there suggestions you can make to help them improve their IPM practices-for weeds as well as insects?
- Do they have possible solutions you could use on your farm?
- Is there a question you have about why or how they answered the way they did?
Files to Download
Module 8 Summative Assessment Worksheet [50]
Submitting Your Assignment
Submit your response in Module 8 Summative Assessment Discussion in Canvas.
Summary and Final Tasks
Summary
Scientists have identified and continue to study and develop strategies to reduce the impact of pests in agriculture. Pest species that are subject to one or few pest control practices over time inevitably develop resistance to the strong selective force. Multiple biological factors and ecological processes, however, influence host-pest population interactions, providing many opportunities to combine pest control tactics and identify new pest control approaches. Climate change will also pose new pest challenges. Some of these challenges are discussed in the online resource that you read parts of in Modules 4 and 5. We highly encourage you to read this a short summary of some of the research on Climate Change Impacts in the United States [51]. See Section title: Key Message 2: Weeds, Diseases, and Pests.
Reminder - Complete all of the Module 8 tasks!
You have reached the end of Module 8! Double-check the to-do list on the Module 8 Roadmap [52] to make sure you have completed all of the activities listed.
References and Further Reading
Benbrook. C. 2014. Impacts of genetically engineered crops on pesticide use in the U.S. -- the first sixteen years. Environmental Sciences Europe 2012, 24:24. doi:10.1186/2190-4715-24-24
Duke. O. S. and S. B. Powles. 2009. Glyphosate-Resistant Crops and Weeds: Now and in the Future AgBioForum, 12(3&4): 346-357
Gassmann A.J., Petzold-Maxwell J.L., Keweshan R.S., Dunbar M.W. 2011. Field-evolved resistance to Bt maize by western corn rootworm. PLoS One. [53] 2011:6(7):e22629. doi: 10.1371/journal.pone.0022629. E pub 2011 Jul 29.
Gassman, A. J. 2016. Resistance to Bt maize by western corn rootworm: insights from the laboratory and the field. Current Opinion in Insect Science. 15: 111-115. doi.org-10.1016/j.cois.2016.04.001.
Georghiou. G. P. 1986. The Magnitude of the Resistance Problem. Chapt 1. 14-44. In Pesticide Resistance: Strategies for the Management. Eds. Committee on of Pest Populations; Board of Agriculture, National Research Council.
Gunsolus, L. J. Weed Science, Department of Agronomy and Plant Genetics. Herbicide-resistant weeds [32].
International Survey of Herbicide Resistant Weeds [38].
Jakka, S. R. K., R.B. Shrestha, and A. J. Gassmann. 2016. Broad-specture resistance to Bacillus thuringiensis toxins by western corn rootworm (Diabrotica virgifera vergifera). Scientific Reports. 6:27860. doi:10.1038/srep27860.
Liebman, M. and E. R. Gallandt. 1997. Many little hammers: ecological management of crop-weed interactions. Pages 291–343 in L. E. Jack- son, ed. Ecology in Agriculture. San Diego, CA: Academic.
Odum, E. P. 1997. Ecology: A Bridge Between Science and Society. Snauer Associates: Sunderland, MA.
Stern, V. M., Smith, R. F., van den Bosch, K. & Ragen, K. S. 1959. The integration of chemical and biological control of the spotted alfalfa aphid: the integrated control concept. Hilgardia 29:81-101.
Tabashnik B., Brevault, T., Carriere, Y. 2013. Insect resistance to Bt crops: lessons from the first billion acres. Nature Biotechnology 31: 510-521.
Additional Reading:
- FAO UN More About IPM [54]
- Cornell University’s Pesticide Safety Education Program (PSEP) [55] Part of the Pesticide Management Education
- Cullen, E., and R. Proost, D. Volenberg. 2008. Insect Resistance Managment and Refuge Requirements for Bt Corn [36]. University of Wisconsin Extension. Pest and Nutrient Management Program.