Let's take our simple cubic crystal structure of eight atoms from the last section and insert another atom in the center of the cube. This new structure, shown in the figure below, is referred to as body-centered cubic since it has an atom centered in the body of the cube. Some examples of metals that possess this crystalline structure include the α phase of iron, chromium, tungsten, tantalum, and molybdenum.
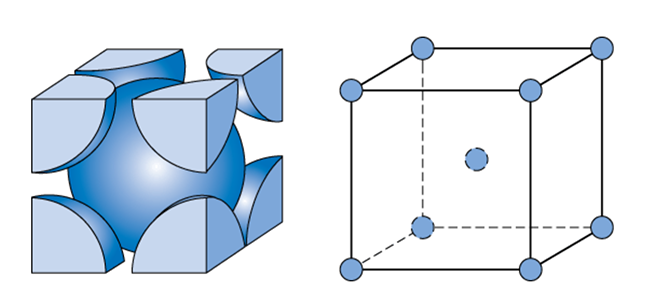
Body-Centered Cubic Structure
Credit: Callister & Rethwisch 5e