There is no such thing as a perfect crystal. Crystalline imperfections (or defects) are always present. In addition, impurity atoms are always present. Many of the properties of materials are sensitive to the presence of imperfections, and not necessarily in an adverse way.
So, what kind of imperfections exist in solids? One way to classify imperfections is by their dimensionality. Point defects exist by definition as a point (0 – dimensional) and include vacancies, interstitial atoms, and substitutional impurity atoms. These point defects are shown in the two figures below and will be discussed further in the reading.
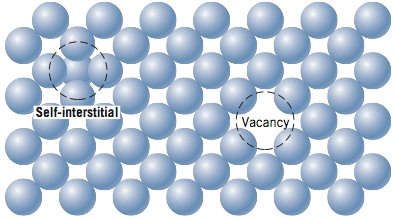
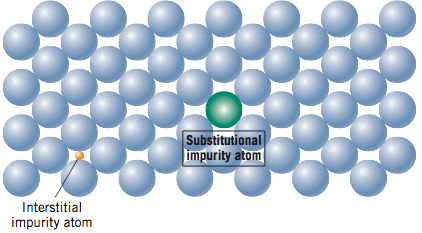
One-dimensional or linear defects are called dislocations. An edge dislocation is when a half plane of atoms disrupts the overall crystal structure. A screw dislocation is when a half twist disrupts the overall crystal structure. A mixed dislocation is a dislocation that combines both an edge and screw dislocation together.
Grain boundaries are regions between different grains within a material. They are classified as an interfacial defect and are two-dimensional.
Now proceed to the second chapter of the Lesson 5 reading assignment and complete the reading.