Silica (structure shown in the top figure below) has a melting temperature of 1700 °C. This is considerably higher than temperatures that are possible with charcoal and a blow pipe (800 - 1200 °C). But adding sodium changes things drastically. Sodium bonds to only one Si atom, so it breaks the ordered network of silica (see lower figure below). This results in a shortening of bond lengths which reduces the melting temperature to around 1000 °C, which is possible to reach with charcoal and a blow pipe. The effect of adding sodium to silica to lower the melting temperature appears to have been discovered around 1,500 BCE.
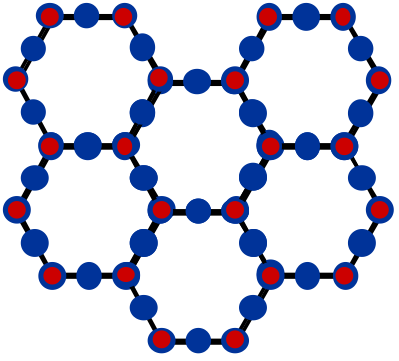
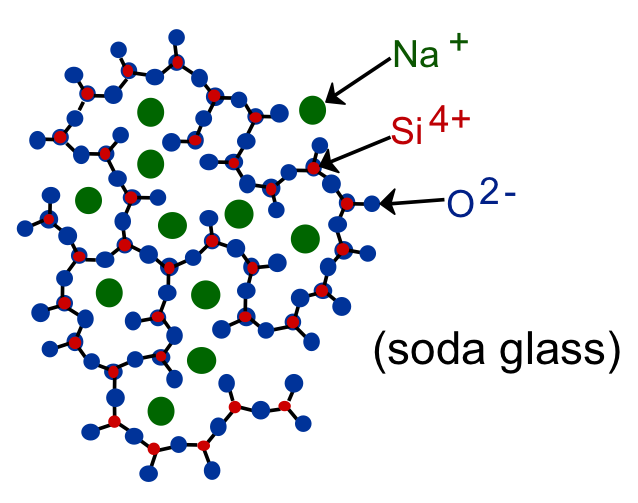
In the 1st century BCE, the Romans developed glass blowing (which has remained relatively unchanged since that time) and the production of glass products increased. Glass is easier to produce than glazing products. An interesting side note is that the Romans recycled glass. In the next section, we'll discuss the bonding of ceramics and compare it to metallic bonding.