Now that you have finished the reading for this lesson, I would like to review the four possible electron band structures for solid materials, as well as p-n junction electrical behavior. In addition, we will define nanotechnology and explore one possible application of nanotechnology which might allow for the continuing improvement in microprocessor speed in coming decades.
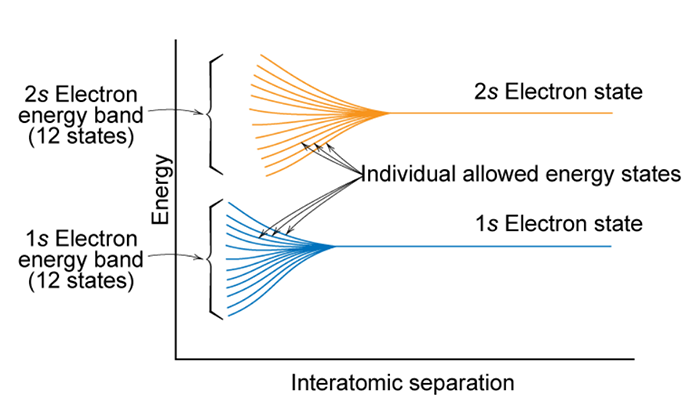
So, what happens when you try to shove a large number ( >1023) atoms together to make a solid? When atoms are separated, electrons will tend to occupy the lowest available discrete energy states. When atoms are brought together, the electrons are forbidden by the Pauli exclusion principle of having identical energy and quantum numbers. As shown in the figure below, as the atoms are brought closer and closer together individual allowed energy states start to spread in energy.
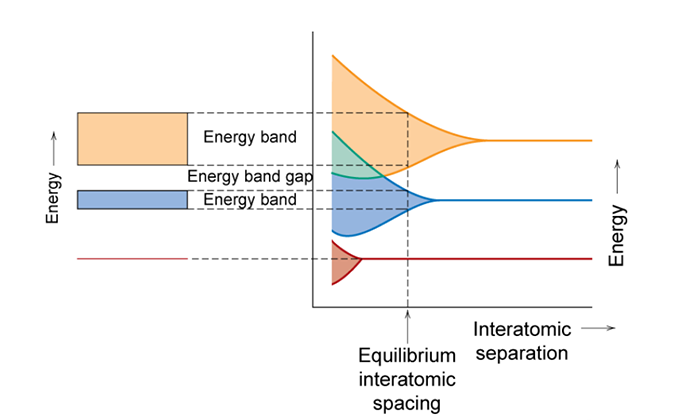
When you bring ~1023 atoms together to make a solid, the separation between the allowed energy states becomes indistinguishable (too small for us to measure). Since we can no longer distinguish the individual states, the allowable energy states form a range of energies that electrons can occupy. The ranges of allowable energies are referred to as bands. These bands are shown in the figure below. In this figure allowable energy levels are plotted versus interatomic separation. However, since there are too many levels to distinguish the band splitting is represented by a shaded region, instead of the individual levels shown in the previous figure. At the equilibrium interatomic spacing, i.e. the average distance between atoms in the stable material, the lowest energy states are not spread. These are 1s electrons of the atoms which are the electrons of the atom that are close to the nucleus of the atom. As such, they do not interact with the other atoms 1s electrons and do not have to spread in energy to satisfy the Pauli Exclusion Principle. However, for this atom at the equilibrium interatomic spacing depict in the figure below, the 2s and 2p states show clear energy splitting. The range of energy splitting for the 2s and 2p states are shown to the left of the graph as energy bands along with the 1s energy level.