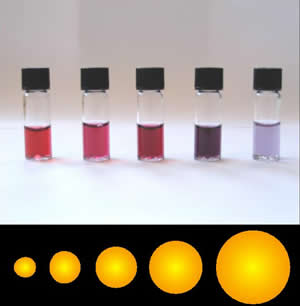
Nanotechnology involves the control of atoms and molecules to produce materials in the size range of 1 – 100 nm, whose size or geometry dominates their material properties. Nanotechnology occurs in a size range were quantum mechanics dominate, but the materials are larger than a single atom. This size range is the range where single-atom behavior is transitioning to bulk material behavior. This allows for the tuning of properties to desirable results, which allows for the creation of designer materials. An example of this is gold nanoparticles. As shown in the figure below, nano-sized gold particles range in color from bright red, pink, purple, to blue, depending on the size of the nanoparticles.
So, how many atoms are we talking about when we say the material ranges from 1 to 100 nm in size?
A cube of 1 nm on a side would have around 100 atoms, while a cube of 100 nm on a side would have around 100 million atoms. That is quite a range. A former professor of this course, Dr. Peter Thrower, in his textbook Materials in Today’s World, calculated how many atoms would be on the surfaces of cubes of 1 nm (nanocube) and 1 cm (bulk cube). What he found was that in the nanocube, 60% of the atoms were on the surface of the cube. This was in stark contrast to the bulk cube, where only one out of 109 atoms were on the surface.
What does this mean chemically? Bulk gold is highly unreactive; it does not tarnish, it does not react with other metals, etc. It is so unreactive that it's possible to find gold in nature in its native state, gold nuggets. Nanogold, on the other hand, is extremely reactive. In bulk gold, the atoms are overwhelmingly non-surface atoms. These non-surface atoms have sufficient neighboring atoms to satisfy their bonds. In nanogold, most of the atoms are on the surface and possess unsatisfied bonds, which makes them extremely chemically reactive. This is a case where the size of the particle matters but also the geometry matters. In the case of nano gold, the geometry of possessing mostly surface atoms results in a chemically active material.