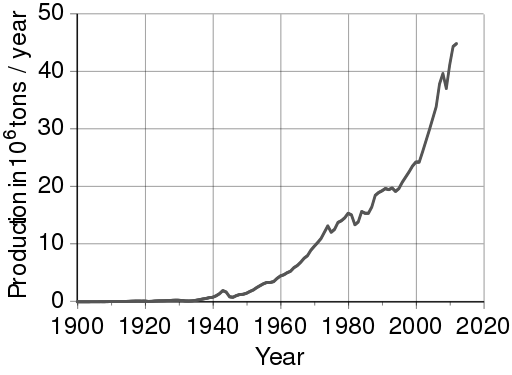
Although aluminum is abundant in nature, it occurs chemically bound to other elements, and there is no known way to smelt aluminum using traditional smelting methods. Because of this limitation, before the 19th century, pure aluminum was rarer than gold. In the 19th century, people learned how to use electrolysis to extract aluminum from aluminum oxide, AlO2. As you can see from the figure below aluminum production has continued to increase ever since.
Typically, aluminum oxide is extracted from the mineral bauxite, and then aluminum is further processed from the aluminum oxide. Visit this website to access the list of where aluminum oxide is produced. Although aluminum oxide is used as an abrasive material most of the aluminum oxide is used for the production of aluminum. For more on the electrolysis process for the extraction of aluminum from aluminum oxide, please watch the fuseschool.org video linked below.
Watch Now
Please watch the following short video (3:13), How to Extract Aluminum Using Electrolysis, on the extraction of aluminum using electrolysis before proceeding to the next section on building lighter aircraft.
Aluminum is the most abundant metal on Earth, however, it is expensive because a lot of electricity is used to extract it. Aluminum conducts heat and electricity well, has a low density, and does not corrode. This makes it very useful for airplanes, drinks cans, electricity cables, and cooking pans. The aluminum ore is called bauxite. Bauxite is purified to yield aluminum oxide which is a white powder. Aluminum is then extracted from the aluminum oxide. The aluminum is extracted by electrolysis. In this video we are going to look at how aluminum is extracted using electrolysis. You should already know how electrolysis works. If you have forgotten, watch our video Electrolysis - How Does it Work, to refresh your memory.
In electrolysis, ions need to pass through the electrolyte and so the aluminum oxide must be made molten so that this can happen. Aluminum oxide has a very high melting point over 2000 degrees Celsius, so instead of trying to melt it the aluminum oxide is dissolved in molten cryolite. Cryolite is an aluminum compound with a much lower melting point than aluminum oxide, and so using this reduces some of the costs in extracting aluminum. The steel case is coated with graphite providing a negative cathode. The positive anodes are immersed in the molten cryolite and are also made of graphite. Remember that graphite is a form of carbon. When the battery is turned on and electricity flows the aluminum from the aluminum oxide in the cryolite forms at the negative cathode and sinks to the bottom of the tank. Here it can then be tapped off as a pure liquid metal. The aluminum sinks because it is more dense than the aluminum prior light solution. The oxygen from the aluminum oxide in the cryolite forms at the positive anodes. The oxygen reacts with the carbon of the graphite forming carbon dioxide. The positive anode therefore burns away and needs replacing regularly. This is another reason for the extraction of the aluminum being so expensive. The overall reaction is aluminum oxide to plus oxygen.
Let's have a quick look at the reactions at the electrodes. At the negative cathode where the aluminum forms the aluminum ions from the molten aluminum oxide cryolite solution are reduced. This means they gain electrons. At the positive anode, where the oxygen reacts with carbon to make carbon dioxide, the oxygen ions are oxidized. This means they lose electrons.
So, from this video you should know that extract aluminum electrolysis is used. Aluminum oxide needs to be molten for the ions to move through it, and so is dissolved in cryolite to lower the melting point. The anode is gradually ward away because the oxygen from the solution reacts with the carbon of the graphite anode producing carbon dioxide, and so the anode wears away and needs to be replaced regularly. Aluminum extraction is very expensive because a lot of electricity is needed.