Sorption is the adhesion of chemicals to solid surfaces. Adsorption process occurs in many natural and engineered systems. Studies of contaminated systems have shown that sorption–desorption is an important geochemical process that regulates transport and fate of inorganic and organic contaminants in natural subsurface systems. For example, metals (Cd2+, Cr3+, Co2+, Cu2+, Fe3+, Pb2+ or Zn2+) can become immobilized by sorbing on sediments and soils. They can also become mobilized through desorption from the solid surface and re-enter the aqueous phase when geochemical conditions allow. Sorption-desorption is widely used in industrial applications including charcoal activation, air conditioning, water purification, among others.
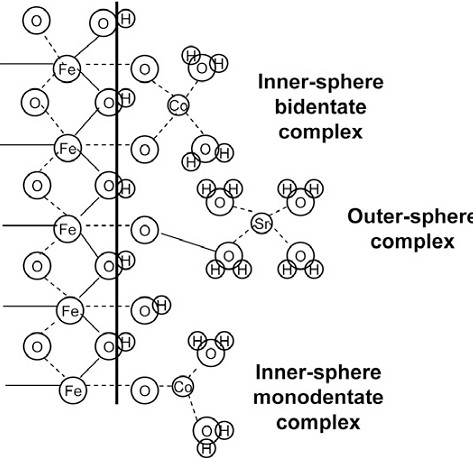
Sorption can occur either specifically or non-specifically as shown in Figure 1. Chemical sorption (Specific adsorption) is highly selective and occurs only between certain adsorptive and adsorbent species. A chemical bond involves sharing of electrons between the adsorbate and adsorbent and may be regarded as the formation of inner-sphere surface complexes. Chemical adsorption is difficult to reverse because of the strength of the formed bond. Physical adsorption (nonspecific adsorption). A physical attraction resulting from nonspecific, relatively weak Van der Waal's forces. Being only weakly bound, physical adsorption is easily reversed. Multiple layers form through outer-sphere surface complexes during physical adsorption [Goldberg 1991; Webb, 2003].
Sorption via surface complexation has been extensively studied. Surface complexation is the process where species in the aqueous phase form complexes with functional groups on solid surfaces, similar to aqueous complexation in lesson 1. Surface complexation function occurs between aqueous species and functional groups on solid surface, instead of the formation of complexes between aqueous species in aqueous complexation reactions. Surface complexation models use mass action laws that are analogous to aqueous geochemical conditions and solid phase properties.