Basic Chemistry and Sources
As we have learned, volatile Organic Compounds (Hydrocarbons) combine with nitrogen oxides (NOx) in the presence of sunlight to form ozone.
In turn, sunlight and hot weather cause ground-level ozone to form in harmful concentrations in the air. As a result, it is known as a summertime air pollutant.
Many urban areas tend to have high levels of "bad" ozone, but even rural areas are also subject to increased ozone levels because wind carries ozone and pollutants that form it hundreds of miles away from their original sources.
View the graph below to compare the major sources of NOx and VOC that help to form ozone.
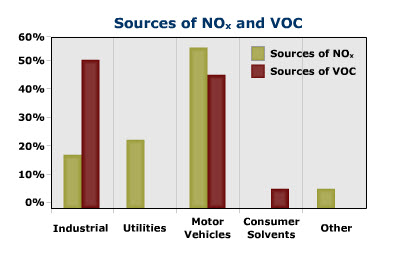
Major sources of NOx and VOC.