Ozone is a secondary pollutant that forms from the primary pollutants such as Volatile Organic Compounds (Hydrocarbons) and nitrogen oxides (NOx) in the presence of sunlight. Its formation is mainly from the automobile emissions.

Below is a demonstration on how ozone forms at the ground level (note ground level ozone is also known as “bad” ozone). Please watch the following 5:29 video:
This demonstration is basically looking at how ozone forms or how photochemical smog forms at the ground level because of the pollutants that we emit.Ozone emission or ozone formation depends on two ingredients, nitrogen oxides that are emitted by pollutants that are going out to the tail pipe of a car and in a power plant with a stack. Nitrogen oxides react with volatile organic compounds. Volatile organic compounds are also emitted by the tailpipe of the cars and some of them are coming from petroleum refineries and petroleum industries. VOCs + NOx = Ozone. These volatile organic compounds react to form ozone only in the presence of sunlight. That is the critical thing, only in presence of sunlight. So, to see this demonstration, we need sunlight. Obviously, we don’t have the sunlight here but I can create sunlight or the wave lengths that are important to create the same effects. That is the reason I am using a UV lamp. You see this lamp here? This blue light is producing the UV light that is required to create ozone. And this ozone again reacts with volatile organic compounds to produce photochemical smog. So first of all I am trying to create here ozone. The way I am creating ozone is not with Nitrogen oxides and volatile organic compounds but turning the oxygen that is there in the beaker into ozone by using this UV light. So let me close this and in about three or four minutes, the oxygen that is there inside will turn into ozone.So we have one ingredient. The second ingredient that is required is Nitrogen oxides or volatile organic compounds to produce smog. Once ozone is formed, I can introduce one of those ingredients and we can see the smog. Ok, I guess we have probably enough ozone in there.Now we need to add Nitrogen oxides and volatile organic compounds to form the photochemical smog that we are seeing or that we should be seeing. Ozone + VOCs + NOx = Photochemical Smog. Since I don’t have my car with me or the tail pipe, I need to somehow produce hydro-carbons in this room.So what I am doing is hydro carbons and Nitrogen oxide, cause even is nitrogen oxides are not there, we can form photochemical smog if we have hydro carbons. The composition will be slightly different. So now I am taking my orange, this is the orange, and you know the beautiful smell that you normally smell with an orange is because of linolenic acid. Linolenic acid is the orange smell that you get.And I’m just taking a small peel of this orange, this is the source of hydrocarbons, and I am trying to put it in here - nothing big deal.And this ozone that is there inside will slowly react with this hydrocarbon that are produced, that are emitted from this orange peel and produce the photochemical smog. Do you see anything going on here? Are you able to still see through the beaker? Is the atmosphere clear in the beaker or is it becoming hazy? Let me turn this little bit around and you may be able to see now, I don’t know. Can you see anything coming out of this? That is the photochemical smog actually. Let me open this and show you. That is the smog that you see and when the smog or when this smoke kind of smog is in the beaker or in the atmosphere, you can not see through and since this has ozone in it, it is not good for our health. When you live in cities filled with this kind of smog, you generally experience breathing problems and other health problems. Your eyes start to water when you drive home from work late in the afternoon or your nose starts to run because your body tries to get rid of, get rid of these pollutants. So that is photochemical smog and if we keep this orange peel like this we probably can generate enough smog for about 24 hours to 36 hours like this, with this little orange peel.Now you can see what we are doing to the atmosphere by emitting roughly 10, 12, 13 million tons of nitrogen oxides into the atmosphere and several million tons of volatile organic compounds into the atmosphere.
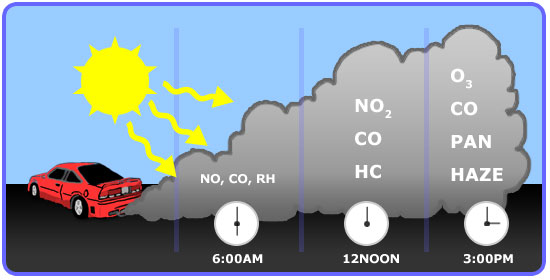
As previously mentioned, the formation of ozone is mainly from automobile emission. A typical profile of pollutants in the air of major cities is well repeatable and is shown in the figure below. Note how the formation changes over the course of a day:
- Early in the morning - During peak traffic hours, NO and Hydrocarbons are emitted along with CO.
- Mid-morning - NO is slowly oxidized to NO2.
- Mid-afternoon - In the presence of sunlight, NOx react with VOCs to form ozone.
Ozone, by itself, is damaging to health and also to the environment. Ozone triggers a variety of health problems even at very low levels and may cause permanent lung damage after long-term exposure. Ozone also leads to the formation of smog or haze, causing additional problems such as a decrease in visibility as well as damage to plants and ecosystems.