Lesson 1.2: What About the Fuel Minerals?
In the last lesson, we learned about the many mineral commodities that are necessary to, and ubiquitous in, modern society. We looked at a few of the 85 or so that are mined in this country, and we learned where to find additional detail on many more of these nonfuel minerals. Fuel minerals are another important part of the mining industry.
The fuel minerals are commonly considered to be coal, uranium, oil, and natural gas. Although oil and natural gas extraction are considered mining for certain purposes, e.g., the U.S. Bureau of Labor Statistics, these two commodities are excluded from discussion in this course.
Uranium is mined and processed primarily as a fuel for use in nuclear reactors, which power steam turbines for the generation of electricity. Uranium is mined by underground and surface mining methods, as well as by solution mining. Currently, within the U.S., there are fewer than ten uranium-mining operations, and all use a form of solution mining; although, in years past, it was mined by underground methods in this country, too. Currently, the world’s uranium supply is largely met by a relatively few number of mines. Australia, Canada, Kazakhstan, Namibia, Niger, and Russia count as the biggest producers, supplying more than half of the global need from less than a dozen mines. We’ll talk more about uranium mining later in this course when we learn about solution mining methods.
Coal most commonly is used as a fuel to generate electricity, i.e., it is burned to create heat to power the steam turbines of electric power plants. In more recent years, the use of all fossil fuels, and particularly coal, has come under attack. Airborne pollutants from coal combustion have been dramatically reduced over the years through technological innovation. However, the CO2 produced with the burning of coal is a serious concern. The production of coal has declined dramatically in recent years as aging coal-fired power plants have been retired and replaced with natural gas fired plants. The cost of permitting a new coal-fired plant is prohibitive in the U.S., and as such no new coal-plant capacity is anticipated in the U.S.
Globally, coal-fired plants continue to be built, and the use of coal as a major fuel source continues unabated. Even in the U.S., the level of coal consumption is projected to remain relatively level at 700 million tons per year over the next several decades. Hopefully, research will produce badly needed solutions to the problems associated with the combustion of fossil fuels! Policy and politics aside, as mining engineers we are concerned with the sustainable production of the mineral commodities demanded by society; and, accordingly, we will address the design and operation of coal mines along with the mines to recover the nonfuel minerals.
Although many people think of coal, as coal – a black material that is burned to produce heat, it is a remarkably complex material composed of organic and inorganic compounds, containing 76 naturally occurring elements of the periodic table. Nearly 120 different minerals have been identified in coal, 33 of which occur commonly, and eight or so are major constituents. Some of the trace elements, such as silver, zinc, or rare earths may prove to be a valuable resource, whereas a few others such as cadmium or mercury may be hazardous if concentrated.
Coals are the result of vegetation dying, decaying, and collecting in swamps forming peat bogs, and the subsequent application of heat, and pressure over thousands and thousands of years. The types of plants, the type and amount of foreign materials, e.g., minerals that were blown or washed into these swamps, determines the quality of the coal, as does the amount of heat and pressure that were applied to the peat over eons of time. As mining engineers, and at this early career stage, we don’t need to delve too far into coal science. However, there are a handful of basic concepts that lay a foundation for understanding how and why we mine and process coal in specific ways.
The makeup of the coal, i.e., the organic and inorganic compounds, the minerals, and the macerals affect the combustion properties of the coal, and they affect the options that we have, or do not have, to prepare the coal for a given commercial use. The latter, is known as coal preparation or simply coal prep. The organic origins of the coal are contained in the macerals, and the inorganic materials are contained largely in the minerals. The inorganic materials can generally be removed economically through the coal preparation process, whereas the organic constituents generally cannot. For example, sulfur in the minerals can be removed through crushing and gravity separation operations, whereas the organic sulfur cannot be removed.
Let’s take a short side trip here to discuss coal prep, or the broader term of mineral processing. In the case of coal, our interest is to remove certain impurities that would degrade the value of the saleable product. For example, we typically want to remove mineral matter such as pyrites. The pyrites contribute sulfur and ash, both of which are undesirable, and hence we apply a variety of techniques to separate and eliminate these undesirable components. During the mining process, we may have further contaminated the run-of-mine coal with the rock layers immediately above or below the coal seam. We’ll need to remove these contaminants as well, before shipping the saleable product.
In the case of the nonfuel minerals, such as phosphate or gold, for example, these valuable components will be bound in the ore, and it will usually be necessary to utilize a series of chemical and physical processes to liberate and concentrate the mineral of interest, while separating out the undesirable constituents. The goal of mineral processing is to beneficiate the valuable components of the ore, and mineral processing engineers design the plants and processes to accomplish this goal. It is often said that mineral processing begins at the mining face where the ore is extracted, and as such, the mining engineer becomes directly involved in not only the first stages of mineral processing but also in the overall success or failure of the mining operation! We’ll talk more about this when we look at the selectivity of various mining methods.
Anyway, back to coal, and the undesirable constituents. Why are they undesirable? Clays will tend to foul boilers, making such coals of lower commercial value. Pyrite minerals will break down into iron and sulfur during combustion, and then, upon recombination with oxygen, the iron oxide will form an ash residue, whereas the sulfur dioxide will be discharged as a flue gas. The latter is heavily regulated and there is a cost to trap this sulfur and prevent its release into the environment. Other mineral matter will generally remain in the ash after combustion. This ash must be disposed, and because it may contain hazardous trace elements, the disposal may be expensive. Hence, coals that naturally have fewer minerals, or coals that have been cleaned through a coal preparation plant have a greater economic value. Power companies buy coal for its calorific content, and they have little interest in transporting, handling, and burning a low-BTU coal product!
The discussion so far has focused on the largest market for coal, which is a fuel source to power steam turbines to generate electricity. For commercial purposes, we often categorize coals as either metallurgical or thermal coal. The former is used to make coke for use in steel-making, while the latter is burned to produce heat, and is often called steam coal. Metallurgical coal has several additional properties, and fewer coals meet the more rigorous requirements to be considered of metallurgical quality. Consequently, met coals often sell for double or triple that of steam coal.
Two very important characteristics of coal are the percentage of fixed carbon and the calorific or heat value of the coal, and these are used to define the rank of the coal, which is a major determinant of the quality of the coal. Coals are classified by rank in the U.S, and there are four ranks. The USGS describes these ranks as follows.
Rank refers to steps in a slow, natural process called “coalification,” during which buried plant matter changes into an ever denser, drier, more carbon rich, and harder material. The four ranks are:
- Anthracite: The highest rank of coal. It is a hard, brittle, and black lustrous coal, often referred to as hard coal, containing a high percentage of fixed carbon and a low percentage of volatile matter.
- Bituminous: Bituminous coal is a middle rank coal between subbituminous and anthracite. Bituminous usually has a high heating (Btu) value and is the most common type of coal used in electricity generation in the United States. Bituminous coal appears shiny and smooth when you first see it, but look closer and you may see it has layers.
- Subbituminous: Subbituminous coal is black in color and dull (not shiny), and has a higher heating value than lignite.
- Lignite: Lignite coal, aka brown coal, is the lowest grade coal with the least concentration of carbon.
Reference: Schweinfurth, S.P., 2009, An introduction to coal quality, in Pierce, B.S., and Dennen, K.O., eds., The National Coal Resource Assessment Overview: U.S. Geological Survey Professional Paper 1625–F, Chapter C, 16 p.
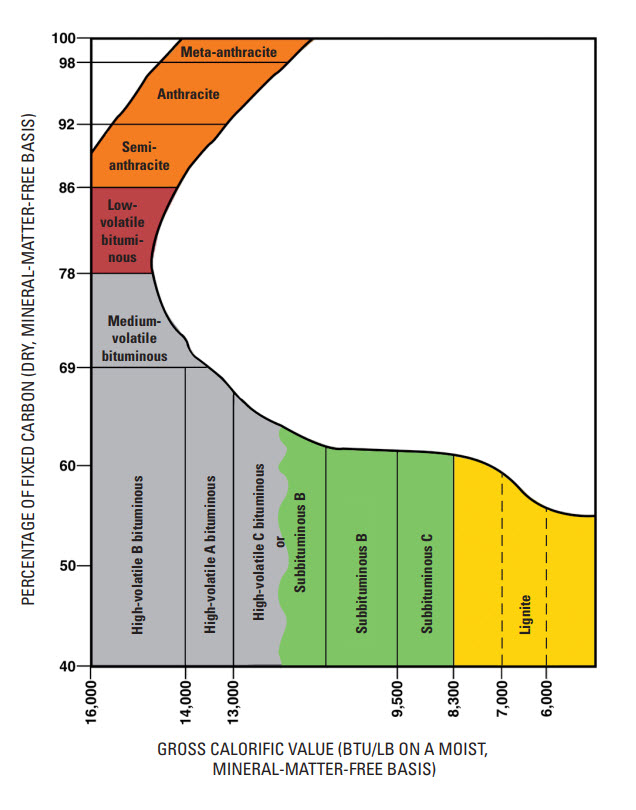
The figure illustrates the rank of the coal in terms of the two characteristics: calorific value and fixed carbon. As a general rule, coals with higher BTU basis are more valuable in the steam coal market, whereas the fixed carbon content, as well as other properties, is more important to the met coal market. Although we usually think of the selling price of steam coal in term of dollars per ton, the utility purchasing the coal is thinking in terms of dollars per million BTU.
Before leaving this brief introduction to coal, it is worth noting the many other uses of coal. Already, we’ve identified one use for coal in addition to the most common use. Do you remember this use? If you said coke for use in steel making, you would be correct. Basically, coke is produced by a destructive distillation process in which the coal is packed into an oven and heated to a high temperature in the absence of oxygen. This drives off the volatile matter, leaving a high carbon product required in the manufacture of steel. The lack of oxygen prevents the volatile matter from burning, and if desired, these components can be recovered. As an example, one ton of bituminous coal roasted in an airtight oven will produce 1300 to 1500 lbs. of coke, 8 to 10 gallons of tar, 3 gallons of light oil, 5 to 6 lbs. of ammonia, and 9500 to 11,000 ft3 of gas! The use of coal to produce synthetic fuels, known as synfuel, has a long history going back to WWII and continuing into the present.
Now, with this new knowledge about nonfuel and fuel minerals, we’re ready to talk about a topic that is near and dear to the hearts of miners… just where do we mine these materials, or put another way, where are those 13,000 mines in the United States?
| Industry | Percent |
|---|---|
| Mining | 15 |
| Other industries | 85 |