For any redox reactions to occur, we need an electron donor and electron acceptor. The oxidation state of the electron donor increases during redox reactions, whereas that of the electron acceptor decreases. In natural environments, organic carbon often serves as the electron donor in microbe-mediated reactions and become oxidized from organic carbon to inorganic form (e.g., CO2), while multiple electron acceptors often co-exist, including oxygen, nitrate, manganese, iron oxides, among others. There are typically multiple functioning microbial groups that use different electron acceptors.
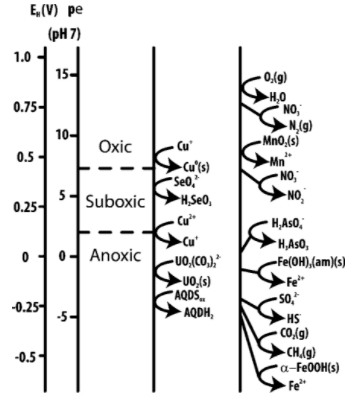
Biogeochemical redox ladder
Microorganisms typically do not use multiple electron acceptors simultaneously. Instead they use electron acceptors in the order of biogeochemical redox ladder, as shown in Figure 1 for some of the naturally-occurring electron acceptors. Different redox reactions release different amount of energy. For example, aerobic oxidation reactions generate much more energy than other redox reactions. The oxidation of glucose can produce 2,880 kJ / mol of C6H12O6; sulfate reduction can produce 492 kJ /mol of C6H12O6, as shown in Figure 2, a much smaller amount. The microorgnanisms that use high-energy-output redox reactions therefore has growth advantage and can outgrow other species.
| Reactions | Chemical Formula | Free Energy kJ/mol glucose |
|---|---|---|
| Aerobic Oxidation | $\mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}+6 \mathrm{O}_{2} \rightarrow 6 \mathrm{CO}_{2}+6 \mathrm{H}_{2} \mathrm{O}$ | -2,880 |
| Denitrification | $5\ \mathrm{C}_6\mathrm{H}_{12}\mathrm{O}_6+24\mathrm{\ NO^-}_3+24\mathrm{\ H}^+\rightarrow\ 30\mathrm{\ CO}_2+42\mathrm{\ H}_2\mathrm{O}+12\mathrm{\ N}_2$ | - 2,720 |
| Sulfate reduction | $6\mathrm{\ C}_6\mathrm{H}_{12}\mathrm{O}_6\ +\ 6\mathrm{\ SO}_4^{2-}+9\mathrm{\ H}^+\rightarrow\ 12\mathrm{\ CO}_2+12\ \mathrm{H}_2\mathrm{O}+3\mathrm{H}_2\mathrm{S}+3\mathrm{HS}^-$ | - 492 |
| Methanogenesis | $\mathrm{C}_6\mathrm{H}_{12}\mathrm{O}_6\ \rightarrow\ 3\mathrm{\ C}\mathrm{O}_2+\ 3\mathrm{\ CH}_4$ | -428 |
| Ethanol Fermentation | $\mathrm{C}_6\mathrm{H}_{12}\mathrm{O}_6\ \rightarrow\ 2\mathrm{CO}_2\ +\ 2\mathrm{CH}_3\mathrm{CH}_2\mathrm{OH}$ | -244 |
*Table modified from Rittman and McCarthy, 2001. Used with Permission
Writing microbe-mediated reactions
Redox reactions written in the form shown in Figure 2, do not consider microorganisms or biomass as part of the reaction products. In order to do so, we need to consider reaction energetics and metabolic pathways. The catabolic pathway breaks down the electron donor (e.g., organic carbon) into smaller molecules and generates energy. The anabolic pathway uses organic carbon and energy harnessed in the catabolic pathway to synthesize large cell molecules. The two pathways complement each other in that the energy released from one is used up by the other. The degradative process of a catabolic pathway provides the energy required to conduct a biosynthesis of an anabolic pathway. Both pathways use electrons from the electron donor. Therefore, electrons from the electron donor partition into the two pathways. To write the full reaction equations, we will need to know the fractions of electrons being used for energy production (fe) and for cell synthesis (fs). The summation of $\ f_e\ and\ f_s\ is\ 1.0$. For different redox reactions, these fractions differ. Those higher in the biogeochemical redox latter generate more energy per electron flow so they need smaller fractions of electrons for energy production (smaller fe) and can channel more electron into cell synthesis. Therefore, values of fe increase and fs decrease as we go from aerobic oxidation that is high in the redox ladder to those lower in the redox ladder. That is, less microbial cells are being produced as we go down the redox ladder with the same amount of electron donor. To write the full reaction equation, we also need chemical formula for microbial cells. A commonly used one is C5H7O2N, approximating the ratios of major elements C, N, O, H, in biomass without including trace elements such as phosphorous, sulfur, metals, etc. Other examples include C8H13O3N2, often used to represent microbial sludge produced in waste water treatment plants. Details of how to write such reaction equations are given in Chapter 2 of Rittmann and McCarthy (2001). Here we show a few examples of reaction equations with biomass as the product using acetate as the electron donor (Cheng et al., 2016; Li et al., 2010). Note that for the text below, the overall reactions R is developed using half reactions of electron donor (Rd), electron acceptor (Ra) and cell synthesis (Rc). The energy production reaction Re = Ra - Rd and cell synthesis reaction Rs = Rc - Rd, respectively. The overall reaction $\ R\ =\ f_e\ R_e+f_s\ R_s=\ f_e\ \left(R_a-R_d\right)+f_s\ \left(R_c-R_d\right)=f_e\ R_a+f_s\ R_c-R_d$. A few examples are shown below.
Aerobic oxidation:
$R_a\ \left(Reaction\ of\ electron\ acceptor\right):\frac{1}{4}O_2+H^++e^-=\frac{1}{2}H_2O$
$\mathrm{R_d\ \left(Reaction\ of\ electron\ donor\right)}:\ \frac{1}{4}\mathrm{HCO}_3^-+\frac{9}{8}\mathrm{H}^++e^-=\frac{1}{8}\mathrm{CH}_3\mathrm{COO}^-+\frac{1}{2}\mathrm{H}_2\mathrm{O}$
$R_c\ \left(Cell\ synthesis\ reaction\right):\ \frac{1}{4}HCO_3^-+\frac{1}{20}NH_4^++\frac{6}{5}H^++e^-=\frac{1}{20}C_5H_7O_2N_{AOB}+\frac{13}{20}H_2O$
Here AOB represent aerobic oxidating bacteria.
The catabolic pathway Re = -Ra - Rd that yields: $\frac{1}{8}CH_3COO^-+\frac{1}{4}O_2=\frac{1}{4}HCO_3^-+\frac{1}{8}H^+$
The anabolic pathway RS = Rc - Rd that yields:
$\frac{1}{8}CH_3COO^-+\frac{1}{20}NH_4^++\frac{3}{40}H^+=\frac{1}{20}C_5H_7O_2N_{AOB}+\frac{1}{20}H_2O$
Combining the two pathways using $R\ =f_e\cdot\left(R_a-R_d\right)+f_s\cdot\left(R_c-R_d\right)=f_e\cdot R_a+f_s\cdot R_c-R_d,$
with $\ f_e=0.4\ and\ f_s=0.6$, we have the following:
$\begin{array}{l}R:0.100\mathrm{O}_2(aq)+0.125\mathrm{CH}_3\mathrm{COO}^-+0.030\mathrm{NH}_4^+\rightarrow\\
0.030\mathrm{C}_5\mathrm{H}_7\mathrm{O}_2\mathrm{~N}_{\mathrm{AOB}}+0.100\mathrm{HCO}_3^-\ +0.090\mathrm{H}_2\mathrm{O}+0.005\mathrm{H}^+\end{array}$
Denitrification
$R a: \frac{1}{5} N O_{3}^{-}+\frac{6}{5} H^{+}+e^{-}=\frac{1}{10} N_{2}+\frac{3}{5} H_{2} O$
$\mathrm{Rd}: \frac{1}{4} \mathrm{HCO}_{3}^{-}+\frac{9}{8} \mathrm{H}^{+}+e^{-}=\frac{1}{8} \mathrm{CH}_{3} \mathrm{COO}^{-}+\frac{1}{2} \mathrm{H}_{2} \mathrm{O}$
$R c: \frac{1}{4} H C O_{3}^{-}+\frac{1}{20} N H_{4}^{+}+\frac{6}{5} H^{+}+e^{-}=\frac{1}{20} C_{5} H_{7} O_{2} N_{N R B}+\frac{13}{20} H_{2} O$
$\text{ Following }R=f_e\cdot R_a+f_s\cdot R_c-R_d,\text{ using a higher }f_e=0.45,\ f_s=0.55,$ we have:
$\begin{array}{l}R:\ 0.090\mathrm{NO}_3^-+0.125\mathrm{CH}_3\mathrm{COO}^-+0.0275\mathrm{NH}_4^++0.075\mathrm{H}^+\rightarrow\\
0.0275\mathrm{C}_5\mathrm{H}_7\mathrm{O}_2\mathrm{N}_{NRB}+0.1125\mathrm{HCO}_3^-+0.1275\mathrm{H}_2\mathrm{O}+0.015\mathrm{N}_2(aq)\end{array}$
Iron reduction reaction
$\mathrm{Ra}:\mathrm{\ FeOOH}(s)+3\mathrm{H}^++e^-=\mathrm{Fe}2^+2\mathrm{H}_2\mathrm{O}$
$\mathrm{Rd}:\ \frac{1}{4}\mathrm{HCO}_3^-+\frac{9}{8}\mathrm{H}^++e^-=\frac{1}{8}\mathrm{CH}_3\mathrm{COO}^-+\frac{1}{2}\mathrm{H}_2\mathrm{O}$
$Rc:\frac{\ 1}{4}HCO_3^-+\frac{1}{20}NH_4^++\frac{6}{5}H^++e^-=\frac{1}{20}C_5H_7O_2N_{\mathrm{Fe}RB}+\frac{13}{20}H_2O$
$\text { Following } R=f_{e} \cdot R_{a}+f_{s} \cdot R_{c}-R_{d}, \text { where } f_{e}=0.60, f_{s}=0.40$
$\begin{array}{l}\mathrm{R}:\mathrm{\ FeOOH}(\mathrm{s})+0.208\mathrm{CH}_3\mathrm{COO}^-+0.033\mathrm{NH}_4^++1.925\mathrm{H}^+\rightarrow\\
\mathrm{R}:\mathrm{\ FeOOH}(\mathrm{s})+0.208\mathrm{CH}_3\mathrm{COO}^-+0.033\mathrm{NH}_4^++1.925\mathrm{H}^+\end{array}$
Sulfate Reduction
$\mathrm{Ra}:\ \frac{1}{8}\mathrm{SO}_4^{2-}+\frac{9}{8}\mathrm{H}^++e^-=\frac{1}{8}\mathrm{HS}^-+\frac{1}{2}\mathrm{H}_2\mathrm{O}$
$\mathrm{Rd}:\ \frac{1}{4}\mathrm{HCO}_3^-+\frac{9}{8}\mathrm{H}^++e^-=\frac{1}{8}\mathrm{CH}_3\mathrm{COO}^-+\frac{1}{2}\mathrm{H}_2\mathrm{O}$
$Rc:\ \frac{1}{4}HCO_3^-+\frac{1}{20}NH_4^++\frac{6}{5}H^++e^-=\frac{1}{20}C_5H_7O_2N_{\mathrm{Fe}RB}+\frac{13}{20}H_2O$
$\text{ Following }R=f_e\cdot R_a+f_s\cdot R_c-R_d,\text{ where }f_e=0.92,\ \ f_s=0.08,$
$\begin{array}{l}R:\ 0.125\mathrm{SO}_4^{2-}+0.13525\mathrm{CH}_3\mathrm{COO}^-+0.004375\mathrm{NH}_4^++0.0065\mathrm{H}^+\rightarrow\\
R:\ 0.125\mathrm{SO}_4^{2-}+0.13525\mathrm{CH}_3\mathrm{COO}^-+0.004375\mathrm{NH}_4^++0.0065\mathrm{H}^+\end{array}$
In each of these reactions, the ratio of the biomass carbon number (C) in the biomass formula (C5H7O2N) produced per C of organic C is called yield coefficient. For example, for aerobic oxidation, the yield coefficient is 0.030*5/(0.125*2) = 0.60; for denitrification, the yield coefficient is 0.0275*5/(0.125*2) = 0.55; for the FeOOH reduction reaction, the yield coefficient is 0.033*5/(2*0.208) = 0.40; for the sulfate reduction reaction, the yield coefficient is 0.004375*5/(2*0.13525) = 0.08 C biomass /C organic. In general, large fs values leads to high yield coefficients. Values of fs are typically in the range of 0.6 – 0.75 for aerobic oxidation, 0.55 – 0.7 for denitrification, and 0.08 – 0.30 for sulfate reduction. For typical values of different types of redox reactions, please refer to Rittmann and McCarthy (2001).