Here we will build upon example 8.1 in lesson 8 to illustrate a magnesite dissolution example in homogeneous and heterogeneous 2D systems based on the experiments in Li et al. (2014). I am reiterating the example 8.1 here.
Example 11.1
The following gives an example of setting up flow and transport calculations in 2D homogeneous and heterogeneous domains. Here we use the physical set up of 2D cross-sections of the Mixed and One-zone column in (Li, Salehikhoo et al. 2014). The authors packed 4 columns of 2.5 cm in diameter and 10.0 cm in length with relatively similar amount of magnesite and quartz distributed in different spatial patterns. Here we focus on two columns that represent two extreme cases: the Mixed column with uniform distributed magnesite and quartz, as well as the One-zone column with magnesite all clustered in one cylindrical zone of diameter of 1.0 centimeter. To keep it simple, here we will do the calculation for 2D cross-sections, instead of following the steps in section 3.2.4 in the paper to convert 2D to 3D. So our numbers might be different from the paper. We are also assuming that in the middle one zone magnesite is 100% of the solid phase and the Mixed column has the same total amount of magnesite as in the One-zone case. The 2D system has a size of 25 mm by 100 mm. A constant differential pressure is imposed at the boundaries in the z (vertical) direction, leading the primary water flow direction in the z direction from the bottom to the top. No flux boundaries are specified in the X direction (Li, Salehikhoo et al. 2014).
Table 1. Physical properties of the columns*
| Columns |
Mg zone |
Qtz zone |
2aL
(cm) |
4aT
(cm) |
ke
(× 10-13m2) |
Φ avg |
| Mixed |
- |
- |
0.05 |
0.005 |
8.26 |
0.44 |
| One-zone |
Width: 1.0 cm
ΦMg: 0.54 |
Width: 1.5 cm
ΦQtz: 0.38 |
0.07 |
0.004 |
10.74 |
0.44 |
*The permeability of the pure sand columns of the same grain size was measured to be 8.7×10-13 m2.
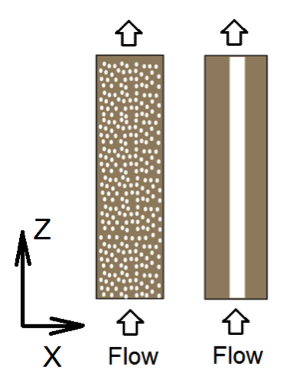
Figure 1. Schematic figures of the 2D cross sections of the Mixed and One-zone columns. The left is for the homogeneous column (mixed) and the right being the One-zone column having magnesite in the mid white zone.
We have shown in the example and video how we calculate the flow field, solute transport, and breakthrough of these two columns. In this lesson we will add the reaction component of this experiment, which is magnesite dissolution. The reaction network and the inlet and boundary conditions are listed in Table 1 and Table 2, respectively. The experiment injected acidic water (inlet) into the two columns, dissolving magnesite. As you probably notice here, the only kinetically-controlled reactions are magnesite dissolution.
Table 1: Chemical reactions and parameters in the column system.
|
Log Keq |
k (mol/m2/s) |
SSA (m2/g)
BET, measured |
| Aqueous speciation (at equilibrium) |
|
|
|
|
|
-14.00 |
- |
- |
|
|
-6.35 |
- |
- |
|
|
-10.33 |
- |
- |
|
|
-1.04 |
- |
- |
|
|
-2.98 |
- |
- |
| Kinetic reactions (logK value is logKsp value) |
|
|
|
|
|
- |
6.20×10-5 |
1.87 |
|
|
- |
5.25×10-6 |
1.87 |
|
|
-7.83 |
1.00×10-10 |
1.87 |
Table 2: Initial and boundary (inlet) fluid composition.
| Species |
Initial Concentrations
(mol/L, except pH) |
Inlet Concentrations
(mol//L, except pH) |
| pH |
8.8 |
4.0 |
| Total Inorganic Carbon (TIC) |
3.43E-3 (Approximate, close to equilibrium with magnesite) |
1.07E-5 (in equilibrium with CO2 gas) |
| Mg(II) |
Varies between 0.52E-5 to 1.20E-5, depending on experimental conditions |
0.0 |
| Na(I) |
1.00E-3 |
1.00E-3 (in dissolution experiment)
1.12E-3 (in tracer experiments) |
| Cl(-I) |
1.00E-3 |
1.00E-3 |
| Br(-I) |
0.0 |
0.0 (in dissolution experiments)
1.20E-4 (in tracer experiments) |
| Si(VI) |
1.00E-5 |
1.00E-4 |
* The measured quantities include pH, total aqueous Mg(II), Na(I), Cl(-I), Br(-I), and Si(VI). Concentrations of all individual species were calculated using the speciation calculation in CrunchFlow based on thermodynamic data in Eq3/6 (Wolery et al., 1990)
In this example, the species from the inlet water include H+, OH-, CO2(aq), HCO3-, CO32-, Na+, Cl-, and Mg2+. Here the major anion is carbonate species so we assume the major aqueous complexes areMgHCO3+ and MgCO3 (aq), as shown in Table 1. This means that we need to solve for 8 (total number of all species) – 5 (total number of secondary species) = 3 governing equations. Note that the ri,tot here should be the total magnesite dissolution rate through the three reaction pathways listed in Table 1:
(2)
Where all rate constants and surface area as shown in Table 1. Please note that in such columns, you can calculate the overall magnesite dissolution rates at the column scale using mass balance of the column:
(3)
Here RMgCO3 is the column-scale magnesite dissolution rates, CMg(II),out is the average Mg(II) concentration coming out of the column outlet, and CMg(II),in is the average Mg(II) concentration coming into of the column. Here please note that we use Mg(II) to represent the total concentrations of Mg(II), instead of individual species such as Mg2+ and MgHCO3+. , where CMg(II),i and qi are the concentration and flow rate from the grid block i in the outlet cross-section. With the same inlet solution across the inlet cross-section, you can directly use the inlet concentration for the calculation.
