Molecular diffusion is driven by concentration gradient and is described by the Fick’s First Law:
Here Jdiff is the diffusive mass flux per unit area (mol/m2/s); D0 is the molecular diffusion coefficient in water (m2/s); and x is the distance (m).
Diffusion coefficients in water are typically in the order of 10-9 m2/s and depend on chemical species, temperature and fluid viscosity. Table 2 lists the diffusion coefficients in water at room temperature for some species. Given the diffusion coefficient at a specific temperature, the diffusion coefficient at another temperature can be calculated as follows:
where $D_{0, T_{0}}$ is the diffusion coefficient (m2/s) at a reference temperature T0 (K), and $\mu_{T}$ and $\mu_{T_0}$ are the dynamic viscosities $\left(Pa\cdot s\right)$ at temperatures T and , T0 respectively.
| Cations | D0 (10-9 m2/s) | Anions | D0 (10-9 m2/s) |
|---|---|---|---|
|
H+ |
9.311 |
OH- |
5.273 |
|
Li+ |
1.029 |
F- |
1.475 |
|
Na+ |
1.334 |
Cl- |
2.032 |
|
K+ |
1.957 |
I- |
2.045 |
|
NH4+ |
1.957 |
NO3- |
1.902 |
|
Mg2+ |
0.706 |
HCO3- |
1.185 |
|
Ca2+ |
0.792 |
HSO4- |
1.331 |
|
Al3+ |
0.541 |
H2PO4- |
0.879 |
|
Fe2+ |
0.719 |
SO42- |
1.065 |
|
Fe3+ |
0.604 |
CO32- |
0.923 |
Fick’s second law combines the mass conservation and Fick’s first law:
where t is the time (s). Eqn. (9) can be analytically solved with appropriate boundary conditions.
Diffusion Coefficient De in Porous Media
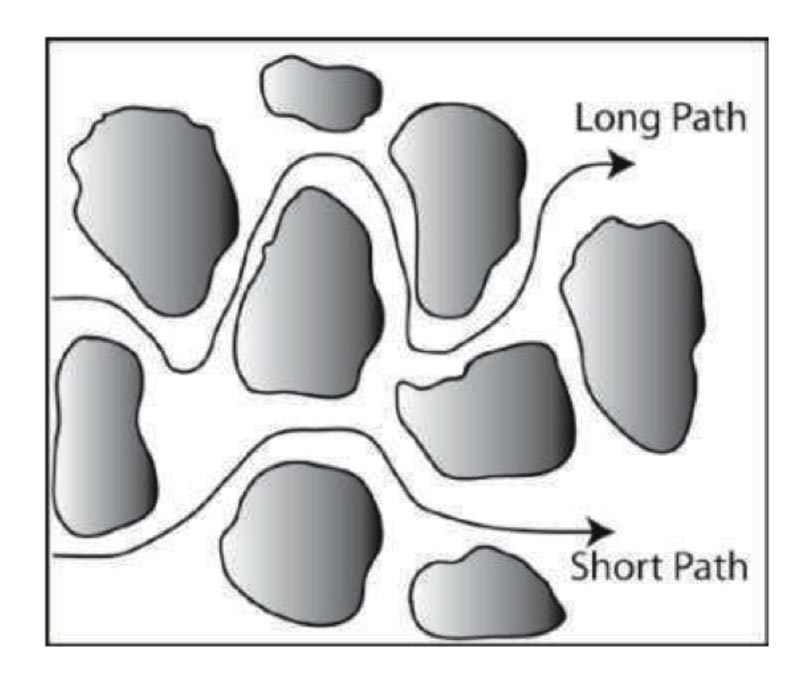
Diffusion in porous media differs from diffusion in homogeneous aqueous solutions. Diffusion in porous media occurs through tortuous and irregularly shaped pores, as shown in Figure 1, and is therefore slower than that in homogeneous solutions. The effective diffusion coefficient describes diffusion in porous media and can be estimated using several forms:
Here F is the formation resistivity factor (dimensionless), an intrinsic property of porous media; the cementation factor (dimensionless) m quantifies the resistivity caused by the network of pores. Reported cementation factors vary between 1.3 and 5.0 [Brunet et al., 2013; Dullien, 1991]. For many subsurface materials, m is equal to 2 [Oelkers, 1996]. TL is the tortuosity (dimensionless) defined as the ratio of the path length in solution relative to the tortuous path length in porous media.