Some of the fuel (hydrocarbon) may not completely burn during combustion and therefore is released into the atmosphere along with the products. The products that are formed during combustion of fossil fuels are shown in the image below:
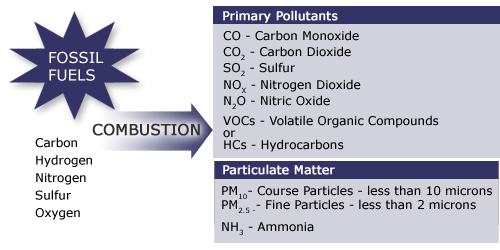
Fossil fuels consisting mainly of carbon, hydrogen, nitrogen, sulfur, and oxygen produce the following products during combustion:
The primary pollutants are Carbon Monoxide (CO), Carbon Dioxide (CO2), Sulfur (SO2), Nitrogen Dioxide (NOx), Nitric Oxide (N2O), Volatile organic compounds (VOCs), and Hydrocarbons (HCs).
The particulate matter produced are Course particles less than 10 microns (PM10), Fine particles less than 2 microns (PM2.5), and Ammonia (NH3).
We will now look at six products of combustion:
- Carbon Dioxide
- Carbon Monoxide
- Sulfur Dioxide
- Nitrogen Oxides
- Lead
- Particulate Matter
Carbon Dioxide (CO2)
Carbon dioxide is one of the maor products of combustion with fossil fuels since carbon accounts for 60–90 percent of the mass of fuels that we burn.
China has emerged as the largest single emitter of energy-related CO2 emissions, surpassing the U.S. in carbon dioxide emissions back in 2010. Now, China emits more than 10 million metric tons while the U.S. hovers around 5 million metric tons. The chart below shows the trend in carbon dioxide emissions since 1980. For Asia and Oceania, and particularly for China and India, emissions can be seen to have increased significantly in the past two decades.
Click the Table, Map, and Chart tabs to see how CO2 emissions have changed over time and around the world:

In 2019, 29 % of CO2 emissions were from transportation, 25 % were from electricity production, 23 % were from industry processes the remaining quarter are from commercial, residential and agricultural applications.
Carbon Monoxide (CO)
If a carbon-based fuel and its products are not completely oxidized (i.e. not burned completely), carbon monoxide will be formed. Carbon monoxide, or CO, is a colorless, odorless gas. The figure below shows the contribution of various sources to the emissions of CO:
Carbon Monoxide is a component of motor vehicle exhaust, which contributes about 55 percent of all CO emissions nationwide. Other non-road engines and vehicles (such as construction equipment and boats) contribute about 22 percent of all CO emissions nationwide. Higher levels of CO generally occur in areas with heavy traffic congestion. In cities, 85 to 95 percent of all CO emissions may come from motor vehicle exhaust.
Other sources of CO emissions include industrial processes (such as metals processing and chemical manufacturing), residential wood burning, as well as natural sources such as forest fires. Woodstoves, gas stoves, cigarette smoke, and unvented gas and kerosene space heaters are sources of CO indoors.
The highest levels of CO in the outside air typically occur during the colder months of the year, when inversion conditions are more frequent. An inversion is an atmospheric condition that occurs when the air pollutants are trapped near the ground beneath a layer of warm air.
Sulfur Dioxide (SO2)
Sulfur dioxide, or SO2, belongs to the family of sulfur oxide gases (SOx). These gases dissolve easily in water. Sulfur is prevalent in all raw materials, including crude oil, coal, and ores that contain common metals, such as aluminum, copper, zinc, lead, and iron.
SOx gases are formed when fuel containing sulfur, such as coal and oil, is burned, and when gasoline is extracted from oil, or metals are extracted from ore. SO2 dissolves in water vapor to form acid and interacts with other gases and particles in the air to form sulfates and other products that can be harmful to people and their environment.
Nitrogen Oxides (NOx)
Nitrogen oxides, or NOx, is the generic term for a group of highly reactive gases, all of which contain nitrogen and oxygen in varying amounts. Many of the nitrogen oxides are colorless and odorless.
Nitrogen oxides form when fuel is burned at high temperatures, as in a combustion process. The primary sources of NOx are motor vehicles, electric utilities, and other industrial, commercial, and residential sources that burn fuels as shown in the figure below.
Although many of the nitrogen oxides are colorless and odorless, one common pollutant, nitrogen dioxide (NO2) along with particles in the air can often be seen as a reddish-brown layer over many urban areas.

Lead (Pb)
The major sources of lead emissions have historically been motor vehicles (such as cars and trucks) and industrial sources.
Due to the phase-out of leaded gasoline, metals processing is the major source of lead emissions to the air today. The highest levels of lead in air are generally found near lead smelters (devices that process lead ores). Other stationary sources are waste incinerators, utilities, and lead-acid battery manufacturers.

A man is pictured in a protective suit as he removes lead-based paint from the US Capitol Dome.

Lead is used in the manufacturing of many items, including
glass, rubber, paint, batteries, insecticides, plumbing, and
protective shielding for X-rays.
Particulate Matter (PM)
Particulate matter (PM) is the general term used to describe a mixture of solid particles and liquid droplets found in the air. Some particles are large enough to be seen as dust or dirt. Others are so small they can be detected only with an electron microscope.
Different sizes of Particles include:
- PM 2.5 describes the “fine” particles that are less than or equal to 2.5 µm (micro meter) in diameter.
- “Coarse fraction” particles are greater than 2.5 µm, but less than or equal to 10 µm in diameter.
- PM 10 refers to all particles less than or equal to 10 µm in diameter (about one-seventh the diameter of a human hair). PM can be emitted directly or formed in the atmosphere.
Different Sources of Particles include:
- "Primary" particles are formed from combustion sources and are emitted directly into the atmosphere. Examples of primary particles are dust from roads or black carbon (soot).
- "Secondary" particles are formed in the atmosphere from primary gaseous emissions. Examples of secondary particles are sulfates formed from SO2 emissions from power plants and industrial facilities; nitrates formed from NOx emissions from power plants, automobiles, and other combustion sources; and carbon formed from organic gas emissions from automobiles and industrial facilities.

The chemical composition of PM depends on location, time of year, and weather. Generally, primary particles make up coarse PM and secondary particles make up most of fine PM.