The Phosphorus Cycle and Human Management of Soils
Basics of The Phosphorus Cycle in Food Production
In an analogous way to the nitrogen (N) cycle on the previous page, we will present the basics of the phosphorus (P) cycle related to food production (refer to figure 5.2.4 below in this section) You'll note that the P cycle is a good deal simpler than the N cycle. For example, there is no gaseous form of P as there is for N, so the atmosphere does not participate in the P cycle. Also, leaching of soluble P is not a major issue as it is for soluble soil N. To begin the description of the P cycle, the large reserve of "primary" P that is accessed by plants and fertilizer production for agriculture is not the atmosphere (as it is for N), but rather so-called phosphate rocks (or rock phosphate) in the crust of the earth, which are mined like other minerals. These rocks are ground up and treated in fertilizer factories to make the phosphate (PO4-) in them water-soluble so that phosphate can be directly taken up by plants from the small pool of soluble phosphorus in soils. In addition to this industrial process that supplies P to plant roots, there are small amounts of soluble P that are continually released by weathering (see Module 5.1) of grains of rock phosphate that form a small part of most soils. These plant-available forms of P from fertilizers and weathering are taken up by plants and pass into the food system when crops are harvested for food products or are fed to livestock. Just as for N (figure 5.2.3), crop residues and manures with organic P are recycled to the soil and are an essential way of replenishing soil organic P supplies. Also, decomposition of soil organic P that liberates soluble P, and uptake of P into the bodies of microbes, link the organic P pool in soil organic matter (SOM) with the small amount of soluble P in soils.
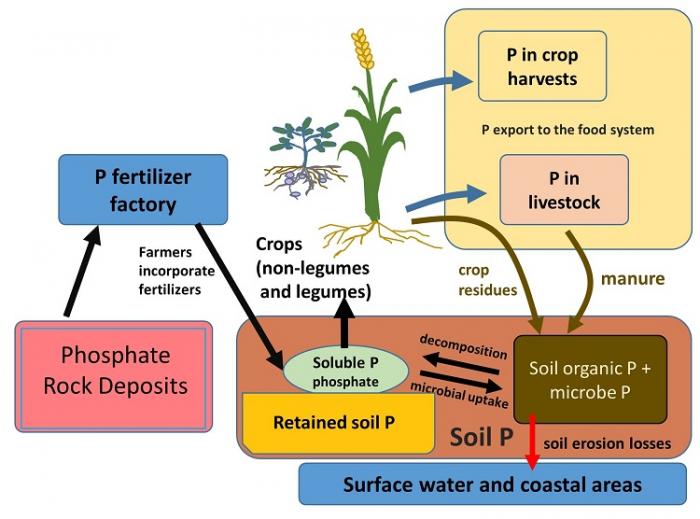
P retention in soils and management responses: "clingy P" versus "flighty N"
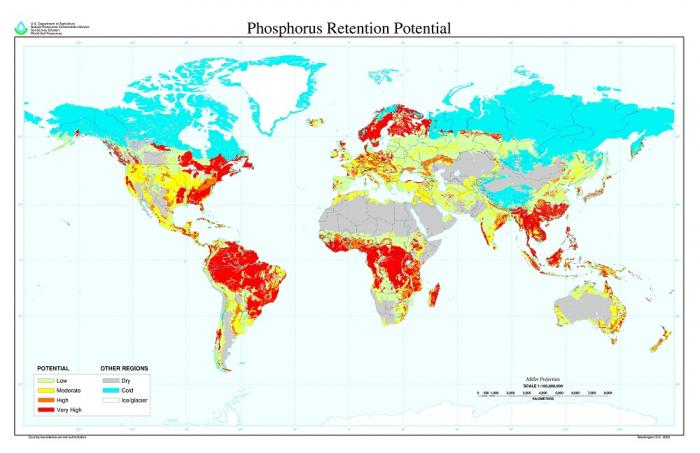
One difference between the cycling of P vs. N in soils is the fact that most soils have ways of chemically capturing and holding soluble P in forms that can become very unavailable to plants. The clay mineral fraction of soils is especially active in retaining P, especially so for the clays that occur in tropical soils (you may be familiar with rusty or yellow-colored clays, made from iron oxides, in warmer areas of the United States and the world). This is called soil retention or fixation of P. In a soil that retains P strongly, less than five percent of the P in applied fertilizer, which enters in a soluble form very suited for plant uptake, is ever available for crops. The rest is quickly locked away by reactions with soil clay minerals. Soil scientists call this process P fixation or P retention, and a global map of estimated P retention has been made (Figure 5.2.5) that summarizes how phosphorus can become limiting to food production, which is a serious problem in many tropical soils. One comparison that may be helpful in remembering the way that soil locks away phosphorus is to contrast it to the behavior of soil N. While soil N is "flighty" or "leaky" with multiple forms and loss pathways, soil P tends to be the "clingy" opposite of soil N -- the issue is not that it is held too loosely in soils but rather that it is held too tightly.
To address the challenge of retained P, farmers may resort to continually supplying fertilizers and manures to crops, often in quantities that greatly exceed crop demand. Nevertheless, additions of organic matter also tend to make retained or fixed P more available, combined with the use of crop species that can better take up fixed forms of P, so that P is moved from the retained, unavailable fraction of P to organic forms in crop and microbial biomass that are eventually recycled into available soluble forms. Certain plant-symbiotic soil microbes, especially mycorrhizal fungi, are particularly efficient at helping plants to access these less soluble forms of soil P. In addition to these soil management measures, first farmers, and now formal plant breeders have developed crop varieties that are more efficient in taking up some of retained P that is locked away in soil.
Soil Erosion and P
As can be seen in Fig. 5.2.4, erosion of particles of soil that contain organic and retained P is the major pathway of phosphorus loss from soils (red arrow in Fig. 5.2.4), in contrast to P export for useful purposes in crop- and livestock-based foods. Along with maintaining the availability of soil P with regard to P retention, protecting soils against erosion is an excellent way to protect the ability of soils to supply P for food production. This main message will be taken up in the summative assessment for this module, and again in Module 7.
Activate Your Learning: phosphorus nutrients required for different foods: a per-acre versus per-person approach
One of the important factors in deciding how much P must be added to soils to replenish them is the amount of P that is exported by typical crops and food products. This exercise will guide you in calculating the amounts of P that leave farm fields on a per area basis, and also at the level of a "phosphorus use footprint" for typical products, analogous to a water footprint in module 4. Consider the table below which reports the use of P to produce unit quantities of a few representative foods. The first column (A) reports very approximately how much a single hectare of soil (100 by 100 m area, about 2.5 acres) will support. The second column (B) is the content or concentration of phosphorus in the food, which means that multiplying A x B gives the kg P exported from the soil by the crop or animal product, which is shown in C. Columns D and E take a slightly different approach: in D the amount of the product eaten by an average U.S. person is reported. In column E, that per-person amount is turned into a per-person consumption of phosphorus in grams (per year)
| Food crops |
(A) kg of fresh product or animal weight sustained per hectare (100 m x 100 m) |
(B) Phosphorus content of the fresh food (g P/ kg fresh wt.) | (C) kg P exported from soil, per Ha (100 m x 100 m) | (D) Per person consumption of product in the U.S. (kg per person per year) | (E) Per capita consumption of soil P resources (g P per person per year) |
|---|---|---|---|---|---|
| carrots | 10000 | 0.35 | 3.5 | 3.2 | 1.1 |
| wheat | 3500 | 7.6 | 27 | 61 | 464 |
| beef | 250a | 7 | 1.8 | 50 | 350 |
| milk | 10000b | 3.6 | 36 | 20 | 72 |
Table assembled by the author from publically available data on typical yields and nutrient content of agricultural products. For example for yield data see National Agricultural Statistics Service (NASS) of the USDA for crop nutrient content see National Resource Conservation Service's Crop Nutrient Database. For nutrient values of foods such as beef and milk see the USDA food composition database.
aAbout the equivalent weight of half a beef cow/steer
bVery roughly a single production cycle (about 12 months) in liters for a single, lactating cow of a high-production variety
