Additional reading from www.astronomynotes.com
Studying blackbody radiation is a useful exercise. However, I have stressed a few times that blackbody radiation is only emitted by an “ideal” or “perfect” radiator. In reality, few objects emit exactly a blackbody spectrum. For example, consider the two spectra you looked at on a previous page: the sun and a blue straggler star. Recall that blackbody radiation is continuous with no breaks. If you look at the two spectra of stars, you see there are black bands in the image of the sun’s spectrum and areas in the plot where the intensity goes to zero or nearly zero in the spectrum of the blue straggler. These gaps in the spectrum where there is no light emitted are called absorption lines. Other astronomical sources (and also light sources you can test in a lab) are found to create spectra that show little intensity at most wavelengths but a few precise wavelengths where a lot of intensity is seen. These are referred to as emission lines.
In the early days of spectroscopy, experiments revealed that there were three main types of spectra. The differences in these spectra and a description of how to create them were summarized in Kirchhoff’s three laws of spectroscopy:
- A luminous solid, liquid, or dense gas emits light of all wavelengths.
- A low density, hot gas seen against a cooler background emits a BRIGHT LINE or EMISSION LINE spectrum.
- A low density, cool gas in front of a hotter source of a continuous spectrum creates a DARK LINE or ABSORPTION LINE spectrum.
You can also summarize Kirchoff's laws in a diagram, like this one:
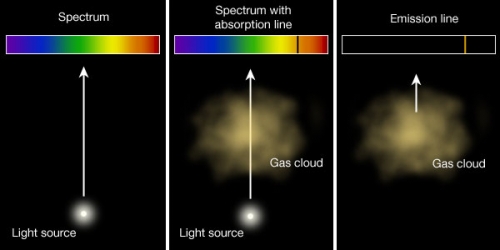
Like Kepler's laws of planetary motion, these are empirical laws. That is, they were formulated on the basis of experiments. In order to understand the origin of absorption and emission lines and the spectra that contain these lines, we need to first spend some time on atomic physics. Specifically, we will consider the Bohr model of the atom.
Whenever you are studying the light from an astronomical object, recall that there are three things you need to consider:
- the emission of the light by the source,
- processes that affect the light during its travel from the source to the observer, and
- the process of detection of the light by the observer.
We observe absorption lines when the light from a background source passes through a cool gas. Somehow, it is the gas that causes the absorption lines to appear in what would otherwise appear to be a continuous spectrum. So, what is going on inside the gas?
A cloud of gas is made up of atoms, which are the smallest components of an element that retain all the properties of that element. A typical cloud of gas in space is likely to contain a lot of hydrogen and helium and trace amounts of heavier elements, like oxygen, nitrogen, carbon, and perhaps iron. The atoms inside the cloud of gas are made up of a nucleus of positively charged protons and neutrons, which have no charge. Surrounding the nucleus are one or more negatively charged electrons. Here is a cartoon image I put together of a helium atom:
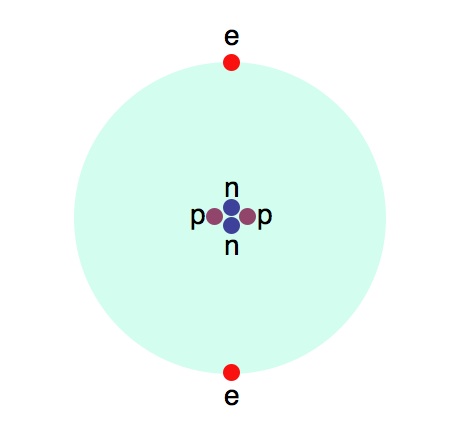
The particles labeled n are neutrons, p are protons, and e are electrons.
Want to learn more?
For a bit more background and information on models of the atom, see a description by the folks at the Jefferson Lab.
Returning to atomic physics and spectroscopy, it is the electrons that are the primary cause of the absorption lines we see in stellar spectra. Bohr proposed a simple model for atoms that required the electrons to occupy “orbits” around the nucleus. The crucial part of his model is to understand that the electrons can only exist in these specific orbits, and not in between. Each orbit has a specific energy associated with it—that is, when the electron is in a specific orbit, it has a specific amount of energy. Thus, the orbits can also be referred to as energy levels. If an electron absorbs exactly the energy difference between the level it is in and any higher level, it can move up to a higher level. Once an electron is in a higher level, it will eventually fall back down to a lower level (either all at once back down to level 1, or by a series of steps down to level 1), and each time it falls from one level to a lower one, it emits a photon that carries exactly the amount of energy equal to the difference in energy between the starting energy level and the ending energy level of the electron. This is shown below. In the top panel, the electrons are falling from higher levels to lower levels and are emitting photons. In the lower panel, the electrons are absorbing photons, causing them to jump to higher levels from their lower levels.
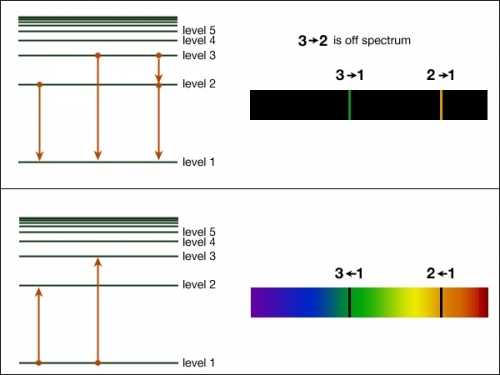
Recall that the energy carried by a photon is given by . So, if the energy of an electron in level 2 is given by and the energy that corresponds to level 1 is given by , then the difference in energy between those levels can be shown as . So, if an electron is in the energy level and falls to the energy level, it will emit a photon with a frequency given by:
,
so, ,
and in this case
giving us
In the top panel above, there is an electron dropping from level 2 to level 1 and emitting a photon with an energy equal to the energy difference between those two levels. So, an astronomer studying the light from that cloud of gas will see an emission line in the spectrum of that cloud with a yellow color, the one labeled "2 - 1" in the spectrum on the right.
Let's tie this idea of electrons moving between energy levels back to the observed spectra of astronomical objects.
Absorption spectra
A continuous source of light emits photons with all different energies. When these photons pass through a foreground cloud (or clouds) of gas, they can encounter the atoms in that gas, each of which has a set of electrons with specific energy levels. Those photons that have precisely the correct energy to kick an electron in an atom of the gas up to a higher level can be absorbed. All those photons that do not have the exact amount of energy to excite an electron pass through the cloud without being absorbed. Thus, what we see after light from a blackbody (that is, the continuous source) passes through a cloud of gas is that most of the photons in a narrow range of frequency (or color) don't make it, leading to breaks, or absorption lines, in the otherwise continuous spectrum of the light source. The absorption lines all correspond precisely to wavelengths or frequencies that are determined by the energy difference between the energy levels of electrons in the atoms that make up the cloud. So, again referring to the energy level diagram above, when an electron goes from level 1 to level 2 by absorbing a photon, an astronomer will observe an absorption line at the frequency that corresponds to that 1 - 2 energy level difference.
Try This!
At the website for the PhET Interactive Simulations, they have a simulation that allows you to investigate models of the Hydrogen atom.
- Go to the Hydrogen atom simulation.
- Click on the play button on top of the image of the simulation to start the simulation. (Note: Your computer / browser may require you to download it rather than play it in the browser).
- In the simulation, use the selector in the top left to choose "Prediction."
- Select "Bohr."
- Turn on the power to the electron gun (click the red button on the drawing) and observe the simulation.
Emission spectra
If you have a low density cloud of gas that is being warmed by some process, the electrons in the atoms in that cloud of gas will not be in the lowest level—they will be in higher levels. So, as they cascade down to the ground level, they will emit photons with precise frequencies, giving rise to emission lines. Neon lights you see in store windows contain low density gas, and the electrons get excited when you run current through the bulb. As the electrons cascade down to the ground level (level 1), they emit emission lines in the red part of the spectrum. Here is an image of a neon containing bulb, and the spectrum it creates when you pass its light through a prism:


A few consequences
Finally, let's end this discussion of spectra with a few consequences of the above physics:
- The energy levels of the electrons in an atom are like fingerprints—no two elements have the same set of energy levels, so the atoms of no two elements create the same pattern of absorption or emission lines. What this means is that if we observe absorption lines caused by a cloud of gas, we can tell what elements make up that cloud by the wavelengths or frequencies of the absorption lines. Tables exist that list all of the known wavelengths of the lines from a particular element as measured in the lab.
- A star will create an absorption line spectrum because the continuous spectrum emitted by the dense, opaque gas that makes up most of the star passes through the cooler, transparent atmosphere of the star.
- The electrons in the gas clouds that create absorption lines should also eventually fall back down to the ground level, so they should also be emitting photons with exactly the same wavelengths as the absorption lines. They do this, but the reason we still observe absorption lines is because the re-emitted photons can be emitted in any direction, while the absorption only occurs along our line of sight.
- When you observe an absorption spectrum of an astronomical object, any cloud of gas between us and the object can absorb light. So, in a typical star, you see absorption lines from the atmosphere of the object, you might see absorption lines caused by intervening gas clouds between us and that star, and finally, Earth's atmosphere will also absorb some of the star's light.