Yes, water cycles (the hydrologic cycle) and geochemical cycles (tracing the paths of various elements to and from seawater). Figure 1 is a conceptualization of the hydrologic cycle (source, USGS). As you are undoubtedly aware, solar energy drives the cycle of evaporation of water from the ocean surface (leaving salt behind), raining out on the continents, and returning to the ocean in rivers (surface runoff). This water does do work on the land surface. Eroding solids and dissolving minerals. Eventually, much of the dissolved material becomes seawater salt. In a geochemical cycle, plate tectonics causes uplift and exposure of "fresh" rocks, which can be weathered by water (and carbon dioxide). Carbon dioxide is driven out of the Earth's interior during volcanism. This is part of the cycle. So, if rock weathering is such an important process, does ocean chemistry simply reflect the chemistry of rivers, only more concentrated? Can the chemistry of the oceans be related to the inputs of rivers alone? We've already examined why water is a powerful solvent, now let's look at the whole picture. The ocean is not simply concentrated river water.
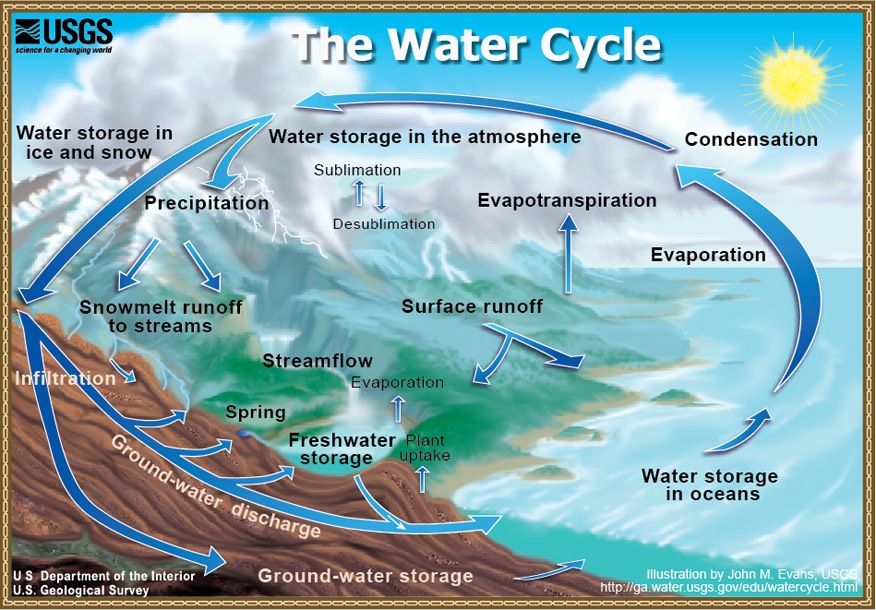
Have you heard Bill Nye's rap version of the Hydrologic cycle? "Water Cycle Jump" Here's a link, but if it doesn't work, please search youtube and find it: Bill Nye the Science Guy - Water Cycle Jump.
In fact, the ocean has a much different chemical composition from average river water because, like water, salts are cycled as well. Some salts build up in seawater over time, while other elements are rapidly used, stripped from seawater into organic matter and skeletons of marine organisms or extraction through alteration of near-seafloor basalt in the midocean ridge hydrothermal system.
Rivers supply a large proportion of dissolved solids to the oceans, but river chemistry is very different from seawater.
In rivers the abundance of elements is HCO3, Ca, SO4, SiO2 (1st 4= 80%), then Cl, Na, Mg, K. Rivers are essentially >35% dissolved inorganic carbon (HCO3 or CO3). Compare this with ocean chemistry in the previous section. The difference in chemical compositions between rivers and ocean reflects sedimentation (precipitation) processes and other inputs/exchanges, such as basalt-seawater reactions at midocean ridges. Activity 2 will help you develop an appreciation of geochemical cycling.
We can examine the "reactivity" of an element in the oceans by looking a the "residence time" of that element--on to the last section...