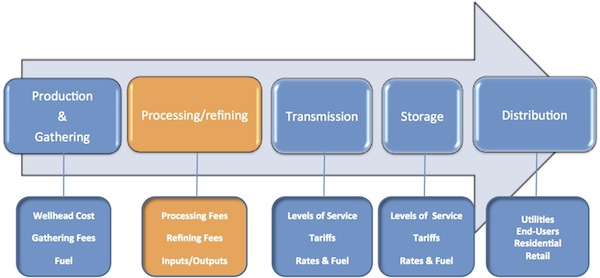
Natural gas that is going to be injected into the pipeline has to meet the pipeline specifications and has to have more than 98% methane. The second step in the logistics chain for natural gas is the processing of the produced gas. Processing is done for two main purposes: 1) removing other heavy hydrocarbons and removing the contaminations. Other extracted hydrocarbons, natural gas liquids (NGLs) and condensates are marketable and can be sold.
The first mini-lecture explains the refining and processing of natural gas. The second one focuses on the NGLs, their applications, and their market.
Key Learning Points for the Mini-Lecture: Processing and NGLs
While watching the mini-lecture, keep in mind the following key points:
- Produced natural gas has to be processed and purified to meet the natural gas pipeline specifications.
- Removing heavy hydrocarbons from natural gas has to be done to protect the burner tip from volatile fuel.
- Produced NGLs are marketable products.
- Water, sulfur content, carbon dioxide, and nitrogen have to be removed from natural gas.
- NGLs include ethane, propane, butane, pentanes, and natural gasoline.
Mini-lecture: Natural Gas Processing (9:04 minutes)
After we've gathered the natural gas from various wellheads and perhaps, brought them to a common point in a gathering system, the next thing that we want to do is kind of twofold. Number one, we want to purify the gas because the gas that goes into the pipeline system has to be about 98% methane with a lot of contaminants removed from that.
But additionally, the processing plants allow us an opportunity to extract natural gas liquids which add to the overall value chain and the actual revenue that can be derived from natural gas.
Here's just a picture of a processing plant up in Colorado. You can see, this is one of the more complex ones.
Now, the basic operations of natural gas processing plants, the first step is to remove the heavy hydrocarbons. Anything that has a specific gravity greater than methane, is considered a heavy hydrocarbon. If you think about it, the raw stream coming out of a natural gas well is going to have a lot of liquid in it. And the liquid happens to be, for the most part, these natural gas liquids. And so we can't have things like propane or butane ending up in someone's hot water heater.
Likewise, in natural gas-fired power plants, they cannot end up with any of these volatile fuels inside a boiler. They literally can have an explosion that occurs there.
And the other side of this, of course, is that we want these heavy hydrocarbons. They are marketable in the form of ethane, propane, butane, isobutane, and natural gasoline.
Simple processes with a processing plant, things like condensation. This is just, you vary the pressure and temperature of the gas stream itself, and then, you can knock out the natural gas liquids. So, for instance, when you heat something up and cool it off, liquids will drop out. If you put something under high pressure and then you reduce the pressure dramatically, liquids will also drop out of the gas stream.
Some of the towers that we'll talk about, they have oils in there which can actually absorb some of the light hydrocarbons. And those then, get funneled off. And then, fractionation is where we actually take the various liquids that may be combined and break them down into individual fractions. Thereby, forming what we call purity products, things like purity ethane or purity propane, which means the majority of that liquid is actually that hydrocarbon.
We want to purify the gas stream as well. Every natural gas pipeline company has certain standards that you can find on their website within their tariff under their statement of operating conditions. You will see that they have certain limitations on things like water, H2S, or hydrogen sulfide, which is corrosive.
Carbon dioxide and nitrogen, they simply take up space in the pipeline so they have no heating value and they just do literally waste space in the pipeline. You'd rather be pushing 98% methane than to have a higher percentage of carbon dioxide or nitrogen for that matter.
So some of the processes would be nitrogen rejection, literally, nitrogen is taken out of the gas stream.
Glycol absorption, now this is ethylene glycol. It's essentially antifreeze and it's heated up, and it can be heated up beyond the boiling point of water. So in essence, the gas stream run through a glycol absorption unit would burn the water off so you reduce the water content in the natural gas stream.
And then, also, if there's a higher level of sulfur than should be in the natural gas stream, they have what's known as an amine treater that will remove that as well.
So now we've purified the gas stream at the processing plant, essentially, we should be left with 98% methane to put into the transmission pipeline that what we call the residue point or outlet of the processing plant.
Some of the general types of processing plants. We have simple separator tower type plants, literally, the natural gas flows through the bottom. And because methane is lighter than the other heavy hydrocarbons, it will rise to the top of the column and then be basically recirculated through the plant.
Then you've got what we call bubble trays on each level and as, again, the gas flow goes through there, the heavier hydrocarbons will settle back down on these trays depending on their specific gravities. And then, again, they are piped off into tanks for storage.
As I mentioned earlier, you're going to vary pressure and temperature with reciprocating compressors. Refrigeration units and so-called re-boilers, the re-boilers are going to heat up the gas stream. Obviously, the refrigeration units are going to cool it down. And the entire time, you're pushing the gas through the processing plant using compressors. So again, raise the temperature of the gas, cool it off rapidly, we'll get condensation, and natural gas liquids will knock out of the stream.
Then we have the next step up and these are the more sophisticated processing plants-- cryogenic or what we call cryo plants. In this case, you're going to cycle the gas through refrigerants using turbine expanders. Turbine expanders in lieu of the type of compressors I was talking about that are jet engines. They're turbine engines.
Again, this idea is to cool it down. Expand the stream using a turbine compressor. And then, when you cool it down, again, you knock out natural gas liquids through the process of condensation.
The idea here, though, is to circulate this gas through the plant several times until essentially, it's been wrung out and you can extract as much NGLs as possible. It's what's called the recovery percentage of the natural gas liquids out of the gas stream.
Here's an overall schematic, and you can see here, you've got some basic-- you start at the wellhead. The oil gas separator that I had in the photo under the natural gas value chain. Condensate separator, that's the heavier liquids that are in-- they are traded in the marketplace similar to oil because they have a lot of crude oil properties.
The dehydration will knock the water out. The next tower takes out the contaminants, the hydrogen sulfide, the CO2. Nitrogen extraction will knock out the nitrogen. A de-methanizer tower literally takes the methane out. You can see when it comes off the top of the de-methanizer tower, it's dry gas. And it goes to the pipeline at the residue part of the processing plant.
And then, the final stage is what's called the fractionator. All these liquids are then broken down into their individual components-- ethane, propane, butane, isobutane, the pentanes which are C5s, and then, your natural gasolines.
And just a quick diagram here based on EIA information that shows the rise in natural gas liquids production over the last several years.
Here are some pictures of-- in the upper left-- the small processing plant and the lower right, a much larger one.
One more thing that we kind of want to talk about here regarding the processing plants is, that processing plants themselves, use some of the natural gas to run their compressors. But also, when you squeeze the natural gas liquids out, you're squeezing out hydrocarbons. You're squeezing out BTU value, heating value.
And so, the amount of gas that you put in, in terms of natural gas, is not going to be the same as what you take out in, again, in the form of natural gas, not necessarily natural gas liquids. So you can kind of see here, we call this plant volume reduction, the volume of BTUs, or the volume of natural gas that comes out on the residue side of a plant, is not the same amount that goes in on the inlet side of the gas.
Now, these are some of the ways that producers and midstream or processing companies put together their contracts. One of the most popular ones is a POP or Percent of Proceeds contract, this is a type of revenue sharing. And so what happens is, the midstream processing company goes ahead and markets the natural gas, and they find markets for the natural gas liquids. And then, they share in that revenue with the producer.
So the producer gets a percent of the net back pricing of the residual gas and the liquids sales, less whatever the midstream company's charging for their processing fees and fuel. So, for instance, we have contracts that might be a 90/10 or an 85/15. Under 90/10, the producer receives 90% of the net revenue and the midstream company receives 10%.
Now there are other producers who prefer to go ahead and market their own natural gas, and what they want then, is what's known as a keep whole agreement. That means that they want the same amount of BTUs that they gave the midstream company on the residue side. And they're going to market it so there's no revenue sharing. And they're going to pay the midstream company some fees for the actual processing.
Mini-lecture: Natural Gas Liquids (9:27 minutes)
And now, we'll talk about some of the specific products that come out, the actual natural gas liquids themselves.
These are hydrocarbon liquids derived from natural gas through the processes that we spoke about. You've got ethane which is C2H6. Propane, C3H8. Butane, C4H10. iso-Butane is an isomer of butane, IC4. The pentanes are what we call C5 pluses, that's C5H12. Natural gasolines, some of them are C5s and some are C6 through C9. And then, condensate is C6 plus. Again, condensate is a very, very light type of oil, and it is marketed in the oil markets.
Again, as we talked about, we've got to remove them from the gas stream itself because of their volatile components, but a lot of these, then, are converted into chemical feedstocks. Some are used as gasoline blending components.
The raw stream coming from most processing plants has to be processed into Y-grade. Y-grade is a composite of all the NGLs that makes it easier to ship it, either by pipeline or for trucks. Then when they arrive at a fractionation facility, that's where they're separated into so-called purity products. And a purity product would contain at least 90% composition of a single natural gas-liquid.
So, for instance, when I talked about purity propane, that would be, the liquid would be at least 90% propane.
The types of NGLs that we have, probably one of the most common ones that we do know is propane. It's approximately 40% of the overall NGL market. It is mostly used for home heating and cooking, but it is also largely used as a chemical feedstock. You can take propane and you make propylene which is a base chemical for plastics.
The primary markets for propane are the Gulf Coast petrochemical facilities. Again, the Gulf Coast is the world's largest refining and petrochemical corridor in the world.
Mont Belvieu, Texas is a large complex east of Houston. It is a huge fractionation facility. There are deliveries to and from the plant. It's a trading point. There's storage there, both above ground and underground. And there's a global market. There are actually NGLs that are exported from this area.
There is a secondary market in the midcontinent. It's in Conway, Kansas. Again, this is a much, much smaller plant than Mont Belvieu, but they have fractionation towers. There is NGL take away and delivery. There is storage there. It is a trading point. And there happens to be a petrochemical refining plant adjacent to the Conway NGL fractionation plant.
And, of course, they do have pipelines that can move southbound to Mont Belvieu so additional NGLs can make their way down to Mont Belvieu.
Ethane is about 25% of the natural gas liquids market. It is primarily used as a chemical feedstock for ethylene and propylene. Again, those are base chemicals used in the manufacture of plastics.
Now, it's rarely used as a fuel source. It can be left in the gas stream as methane. We refer to this as ethane rejection. If ethane prices are low but natural gas prices are fairly strong, then the ethane is left in the natural gas stream which raises the overall BTU content for the stream. And, of course, this is highly price dependent.
Sometimes in the middle of winter, the actual price of the natural gas on a BTU basis far exceeds that of selling the ethane in liquid form. And so you'll find processing plants go into what they call ethane rejection, that means is, they don't want the ethane. They leave it in the natural gas stream.
Same market hubs as the other NGLs, Mont Belvieu and Conway, Texas. If you hear the term E/P mix in the natural gas liquids industry, that means that it's 80% ethane and 20% propane, and that is strictly used for ethylene production.
Butane or n-butane, because now we're talking about the normal butane, 85% of the butane is used for gasoline blending. Now we talk about RVP. In the wintertime, butane is used to stabilize the RVP. RVP is the re-vapor pressure. Now it's a measurement of the ability for gasoline to vaporize at atmospheric pressure.
Any time that you are filling your car up if you see fumes coming back up out of the tank while you're filling it, that's a measure of RVP. You've got vapor there.
Several states in the United States have those vapor recovery nozzles, those plastic things that are over the gas lid. The idea is that you want to recover as much vapor as possible because number one, they do condense and they are gasoline. Number two, they are a form of pollution when they just go to the air.
We talked about the refining process. Butane is used as a cracking component when we talked about the cracking portion of a refinery process. A lot of us know about lighter fluid, meaning butane is used in lighters. It's also a propellant in aerosol sprays. It can be used for household cooking and bottled gas. And it's also a refrigerant. It is a refrigerant that can be used at the processing plants to chill the natural gas. It is also a refrigerant used in air conditioning systems in motor vehicles.
And this is iso-butane which is an isomer of butane, also known as methylpropane. It has similar uses to the butane. Gasoline blending. It's a chemical feedstock. Again, it's also used as a refrigerant in automobiles for the air conditioning systems and it's known as R600a.
And it's also known as isooctane, this is an anti-knock gasoline additive. If you've ever had the situation with knocking your car, seems like it's starting to stall and it bangs really hard and then it shuts down, this helps to stabilize the gasoline so that those things do not happen because they can be very damaging to the engine.
NGLs, the next group of natural gasolines-- C5 pluses are considered natural gasolines. You're literally able to burn those as natural gasolines. So these are also used for gasoline blending. Now they're used to stabilize the RVP mostly in the summertime. They can be used for ethylene production. They are used as industrial solvents. They're also an ethanol denaturant.
If you think about ethanol, the vast majority of ethanol is produced from corn, and it's a form of alcohol. So it literally is corn liquor. You could drink it as an alcohol. So to discourage people from doing that and to prevent the sale of it as that, basically, they have to add a little bit of natural gasoline to it so, in essence, it becomes lethal, and it certainly tastes bad.
It's used as a crude diluent, which means it can be used to dilute crude. For instance, in the Western provinces, especially in Alberta, Canada, you have the tar sands oil which is also known as bitumen, and it's extremely thick. And so they can take the natural gasolines and they can blend those, or add them to the thick crude, which makes it a bit thinner which allows it to more easily ship in the pipelines.
And again, the biggest market hub for natural gasolines is Mont Belvieu, Texas.
Pricing wise, you can see, this is just a comparison of the natural gas liquids which tend to run in sync with things like natural gasolines and crude oil.
And here, you can see just basically, again, another trend where you've got spot prices for natural gas liquids. Compared to Brent crude, Mont Belvieu propane, you can see that that's in here. And then, of course, natural gas.
Now this last slide I want to show you has to do with the fact that you can see the spike of mostly propane, this is propane spot pricing. And you can see in the winter of 2013-2014, there was a huge spike, specifically Conway, Kansas.
Now, this didn't necessarily have to do with the amount of propane, it had to do with the deliverability. We couldn't get the propane to the markets that needed it the most and that was in the Upper Midwest. You can see this past winter of 2014-2015, there was not the same type of spikes in Kansas or at Mont Belvieu.
And then lastly, I've got a slide here that kind of gives you an appreciation of the value of natural gas when you add in the revenue from the liquids. A lot of people want to know, why do producers continue to sell natural gas above or below $3? How is it possibly economical?
Well, if they're also extracting natural gasolines, then you've got a considerable amount of revenue there that's possible so you can see as you move across this spreadsheet and you get over to the liquid price per MMBTU, it is considerable.
And then we talk about the spreads that the midstream or processing companies get, and you can see in this particular sample, the total stream was worth about $3.20. The gas that it cost them to basically run the plant, was $2.65 so their crack spread becomes $0.55 per MMBTU, which is a pretty healthy spread.