4.3 Pretreatment of Lignocellulosic Biomass
When the word carbohydrate is used, I typically think of the carbohydrates in food. Carbohydrates are the sugars and complex units composed of sugars. This section will describe each.
Sugars are also called saccharides. Monomer units are single units of sugars called monosaccharides. Dimer units are double units of sugars called disaccharides. Polymers contain multiple units of monomers and dimers and are called polysaccharides.
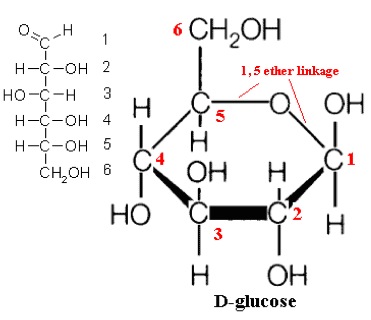
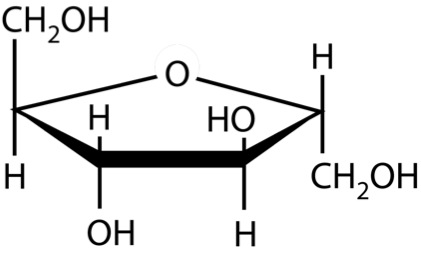
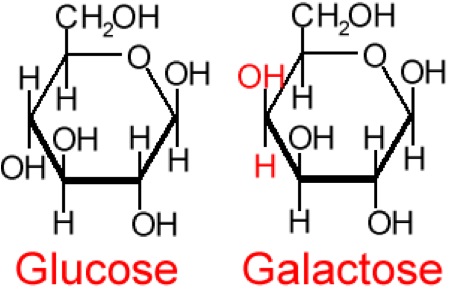
So, what are typical monosaccharides? They are made up of a molecule that is in a ring structure with carbons and oxygen. The first figure below shows the structure of glucose; it is made up of C6H12O6. Glucose is distinguished by its structure: five carbons in the ring with one oxygen; CH2OH attached to a carbon; and OH and H groups attached to the other carbons. This sugar is known as blood sugar and is an immediate source of energy for cellular respiration. The second figure shows galactose next to glucose, and we can see that galactose is almost like glucose, except on the No. 4 carbon the OH and H are an isomer and just slightly different (highlighted in red on the galactose molecule). Galactose is a sugar monomer in milk and yogurt. The third figure shows fructose; while it still has a similar chemical formula as glucose (C6H12O5), it is a five-membered ring with carbons and oxygens, but two CH2OH groups. This is a sugar found in honey and fruits.
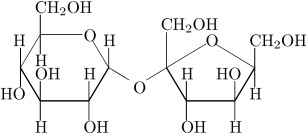
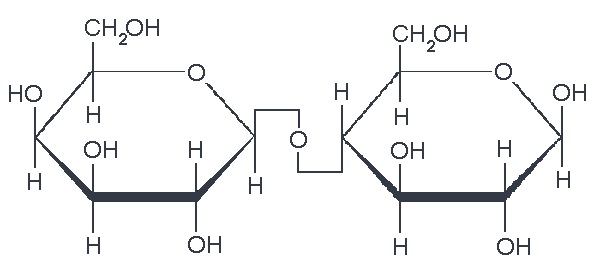
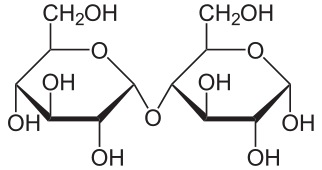
We also have disaccharides as sugars in food. Disaccharides are dimers of the monomers we just discussed and are shown below. One of the most common disaccharides is sucrose, which is a common table sugar. It is a dimer of glucose and fructose. Another common sugar dimer is lactose. It is the major sugar in milk and a dimer of galactose and glucose. Maltose is also a sugar dimer, but is a product of starch digestion. It is a dimer made up of glucose and glucose. In the next section, we will discuss what starch and cellulose are composed of in order to see why maltose is a product of starch digestion.
Carbohydrate structure
All carbohydrate polymers are monomers that connect with what is called a glycosidic bond. For example, sucrose is a dimer of glucose and fructose. In order for the bond to form, there is a loss of H and OH. So, another way to show this is:
C12H22O11 = 2 C6H12O6 − H2O
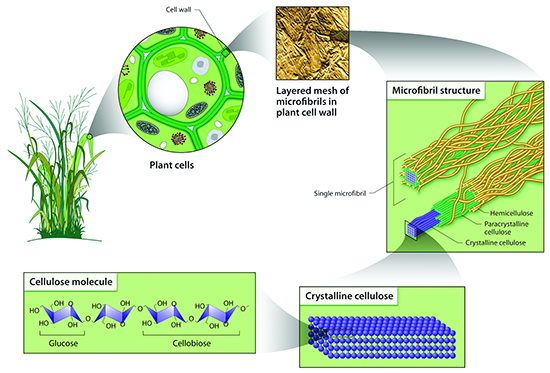
There is a wide variety of sources for lignocellulosic biomass, which includes agricultural waste (i.e., corn stover), forest waste from furniture and home construction, municipal solid waste, and energy crops. They all look very different, but all are composed of cellulose, hemicellulose, lignin, and other minor compounds. The figure below shows switchgrass (with parts magnified to emphasize different parts of the plant structure). Once you get down to the microfibril structure, you can see the components of the microfibril, which include lignin on the outside layer, hemicellulose on the next layer, and finally, cellulose. Because of the structure, the lignocellulose is difficult to break down, which is known as recalcitrance. In order to get to the cellulose, the cell wall has to be opened up, the lignin has to be removed or separated from the hemicellulose and cellulose, and then the cellulose, crystalline in nature, has to be broken down. All these steps are resistant to microbial attack, so pretreatment methods are used to break it apart. In other words, biomass recalcitrance requires pretreatment.
Another Perspective
You can access the following online journal article to see another illustration of lignocellulose but with the lignin component included (Figure 1):
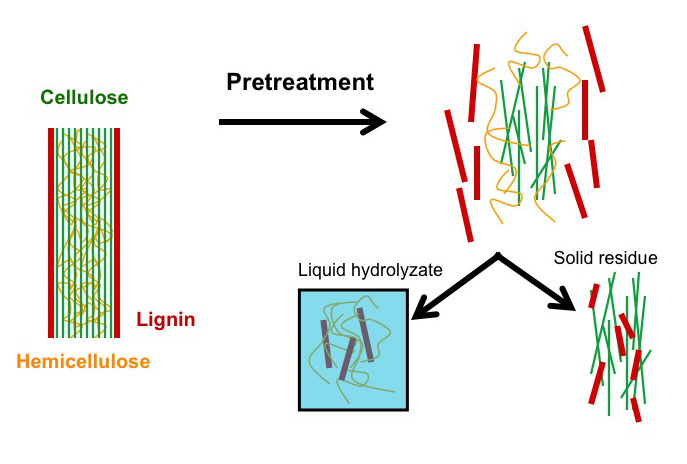
Pretreatment is the most costly step; however, the only process step more expensive than pretreatment is no pretreatment. Without pretreatment, yields are low and drive up all other costs, more than the amount saved without pretreatment. Increased yields with pretreatment reduces all other unit costs. Below is a schematic of the role pretreatment plays. Pretreatment, depending on the method, will separate the lignin, the hemicellulose, and the cellulose. The schematic shows how these break apart. Part of the lignin and the hemicellulose are dissolved in liquid during hydrolysis, and part of the lignin and the cellulose are left as a solid residue. There is a partial breakdown of the polymeric molecules, and the cellulose is now more accessible to microbial attack.
Pretreatment is costly and affects both upstream and downstream processes. On the upstream side, it can affect how the biomass is collected or harvested, as well as the comminution of the biomass. Downstream of pretreatment, the enzyme production can be affected, which in turn will affect the enzymatic hydrolysis and sugar fermentation. Pretreatment can also affect hydrolyzate conditioning and hydrolyzate fermentation. The products made and the eventual final processing also will be affected by pretreatment. However, it is more costly to not do pretreatment.
There are two different types of pretreatment. Physical effects disrupt the higher-order structure and increase surface area and chemical/enzyme penetration into plant cell walls, including mechanical size reduction and fiber liberation. Chemical effects include solubilization, depolymerization, and breaking of crosslinks between macromolecules. The individual components can “swell," depending on the organic solvent or acid used. Lignin can be “redistributed” into a solution, and lignin and carbohydrates can be depolymerized or modified chemically.
The following pretreatment technologies will be discussed in more depth: 1) size reduction, 2) low pH method, 3) neutral pH method, 4) high pH method, 5) organic solvent separation, 6) ionic liquid separation, and 7) biological treatments.