8.2 The Reaction of Biodiesel: Transesterification
So, how do we make biodiesel?
The method being described here is for making FAMEs biodiesel. The reaction is called transesterification, and the process takes place in four steps. The first step is to mix the alcohol for reaction with the catalyst, typically a strong base such as NaOH or KOH. The alcohol/catalyst is then reacted with the fatty acid so that the transesterification reaction takes place. The first figure below shows the preparation of the catalyst with the alcohol, and the second figure shows the transesterification reaction.
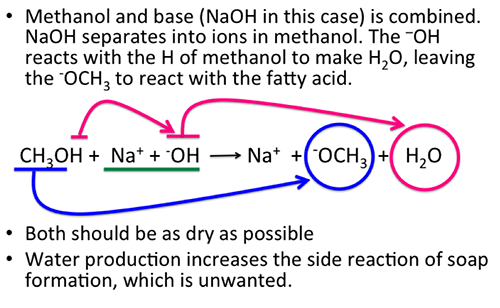
The catalyst is prepared by mixing methanol and a strong base such as sodium hydroxide or potassium hydroxide. During the preparation, the NaOH breaks into ions of Na+ and OH-. The OH- abstracts the hydrogen from methanol to form water and leaves the CH3O- available for reaction. Methanol should be as dry as possible. When the OH- ion reacts with H+ ion, it reacts to form water. Water will increase the possibility of a side reaction with free fatty acids (fatty acids that are not triglycerides) to form soap, an unwanted reaction. Enzymatic processes can also be used (called lipases); alcohol is still needed and only replaces the catalyst. Lipases are slower than chemical catalysts, are high in cost, and produce low yields.
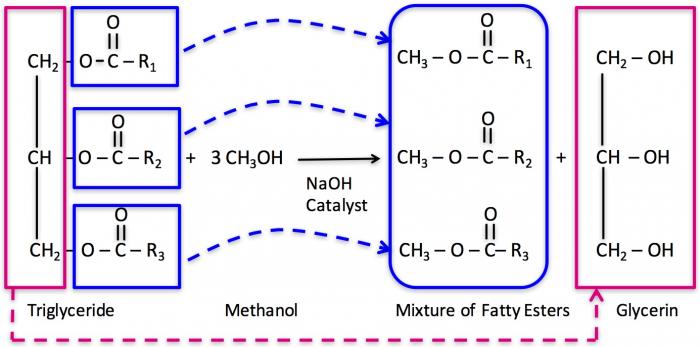
Once the catalyst is prepared, the triglyceride will react with 3 mols of methanol, so excess methanol has to be used in the reaction to ensure a complete reaction. The three attached carbons with hydrogen react with OH- ions and form glycerin, while the CH3 group reacts with the free fatty acid to form the fatty acid methyl ester.
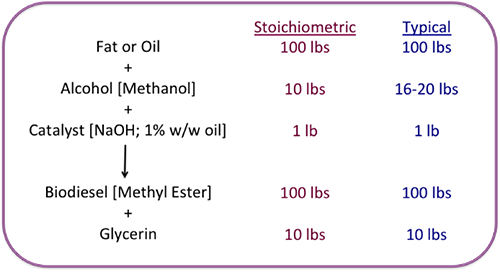
The figure below is a graphic of the necessary amounts of chemicals needed to make the reaction happen and the overall yield of biodiesel and glycerin. The amount of methanol added is almost double the required amount so the reaction goes to completion. With 100 lbs of fat and 16-20 lbs of alcohol (and 1 lb of catalyst), the reaction will produce 100 lbs of biodiesel and 10 lbs of glycerin. The reaction typically takes place at between 40-65°C. As the reaction temperature goes higher, the rate of reaction will increase, typically 1-2 hours at 60 °C versus 2-4 hours at 40°C. If the reaction is higher than 65°C, a pressure vessel is required because methanol will boil at 65°C. It also helps to increase the methanol-to-oil ratio. Doubling the ratio of 3 mols of alcohol to 6 mols will push the reaction to completion faster and more completely.
The following video shows a time-lapsed reaction of transesterification of vegetable oil into biodiesel. It also incorporates the steps after the reaction to separate out the biodiesel (9:44).
MARK HALL: Hello. I'm Mark Hall of the Auburn University Extension Renewable Energy Specialist. We're doing several of these things on energy options that you can do, several pieces that, each piece of the puzzle, that you can contribute to our energy independence by making ethanol, making biodiesel, being more energy efficient in how you operate your home.
Today, we're going to talk about making biodiesel. And we have Lance Hall. Lance has been making biodiesel to run in his car. He bought a used Volkswagen off eBay and started making biodiesel. And he's liked it so much that he's bought a new diesel car. And he's been real successful doing this for a couple of years.
Before we bring Lance in, I'd like to thank my friend and coworker Walter Harris, the county agent coordinator in Madison County, for filming us today. Lance, come in and show us what you've been doing. And congratulations. You've been successful doing this.
I was talking to my daddy about my new job several years ago. And he said, well, Lance has been doing that for a long time. I said, what? I didn't know that. So Lance, show people how to make biodiesel.
LANCE HALL: OK. A lot of people know about the biodiesel. They've read the stories. They've done some research. But, yet, they still don't have enough confidence in their ability to actually make a batch. So I'm going to show you today on how to make a batch of biodiesel, just small scale, but it's easy.
OK. The first thing that we're going to do is start off with vegetable oil. Now, this will be 800 milliliters. And don't be confused between the milliliters and your normal units of measure. It's a simple conversion that anybody can do with a handheld calculator.
So we've got 800 milliliters here. Well, first thing we want to do is heat it. Now, don't be concerned about this fancy piece of equipment, either. The main element of this is to heat it any way that you can safely.
And these things here are magnetic stirrers. Again, don't be concerned with this. Just stir it while you're heating it to even things out. And we're going to heat this up to about 130 degrees Fahrenheit.
MARK HALL: Lance, tell them about where you get this equipment.
LANCE HALL: All of this equipment that I've got in my shop, all my lab stuff, eBay is a wonderful place to find a used lab supplies, lab glass. These are magnetic stirrer plates. These are really handy to have if you have the means to buy them. You don't have to have them, of course. But I like to use them.
And this is also an electronic scale that comes in handy when you start weighing out your catalyst, doing anything that you want to measure a precise weight. That's worth the money there. And that's going to take a little while, so--
MARK HALL: Lance, is there any other sites, internet sites that you would recommend for people that are interested in making biodiesel?
LANCE HALL: There are several sites out there. One of the most informative on what biodiesel is, where it's being used, is biodiesel.org. That's the National Biodiesel Board website, lots of good information there. It won't really tell you as much how to make it, but hopefully, this will be one of the more informative sites that you'll actually be able to see somebody make one, make a batch.
OK. As our oil is heating up, we have to mix up our methanol potassium hydroxide mixture. So safety is paramount with the use of methanol or the strong caustic lye potassium hydroxide. Methanol can cause blindness or death, and it can be absorbed through the skin. And the potassium hydroxide will burn your skin if it gets on you.
So here's what we're going to do. We're going to take our methanol and we're going to pour this into a container. Face shields are good, too.
We're going to use 175 milliliters of the methanol. That's roughly 20% of the 800 milliliters of oil. You usually want to use about a 20% methanol volume compared to the veggie oil volume.
OK. Our next ingredient is our potassium hydroxide. That's our lye. Now, we have to do a quick calculation on how much of this we need to mix with our methanol in order for the reaction to take place.
I've got a nice spreadsheet that I like to use. It's the Biodiesel-o-matic. You can usually find it online from different biodiesel websites. I'm going to pull that up.
OK. We want to use 7 grams of potassium hydroxide per each liter of veggie oil. So you take 7 divided by 0.8. And that gives you 6.4 grams.
Double bag this stuff, or it will absorb moisture. And that will kill your process.
So we're going to use our scale. We're going to zero the container. And then we're going to put 6.4 grams into it. Make sure you have your gloves on.
OK. That's our 6.4 grams. Close this immediately. Keep it double-bagged. OK. Now, you're going to take your 6.4 grams of potassium hydroxide and put that into your 175 milliliters of methanol.
Again, you want to stir this. It's not necessary to heat it, though. Just stir. And stir this until at least the potassium hydroxide is completely dissolved into the methanol. You don't want to see any chunks of white potassium hydroxide flakes.
All right. Our potassium hydroxide is fully mixed into our methanol. We want to remove the stir bar. And then we're just going to slowly pour this into our oil as it's being stirred.
Again, you don't have to have fancy equipment. Just pour it in as you're stirring it manually. But the key is to do it slowly.
The figure below shows a schematic of the process for making biodiesel. Glycerol is formed and has to be separated from the biodiesel. Both glycerol and biodiesel need to have alcohol removed and recycled in the process. Water is added to both the biodiesel and glycerol to remove unwanted side products, particularly glycerol, that may remain in the biodiesel. The wash water is separated out similar to solvent extraction (it contains some glycerol), and the trace water is evaporated out of the biodiesel. Acid is added to the glycerol in order to provide neutralized glycerol.
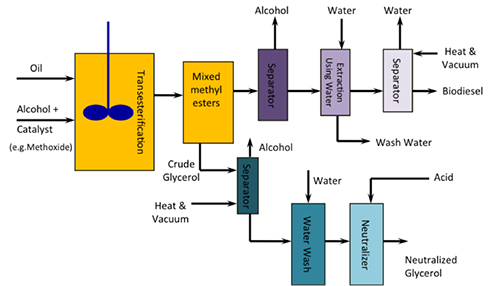
Schematic of the biodiesel process using transesterification:
Oil, alcohol, and a catalyst undergo transesterification. From there they are mixed methyl esters from which crude glycerol is removed. The crude glycerol goes into a separator under heat and a vacuum in which alcohol is removed. It then goes through a water wash and is neutralized with acid to produce neutralized glycerol. The other remaining mixed methyl esters from transesterification go into a different separator which removes any alcohol. They then undergo an extraction using water and move into a second separator under heat and a vacuum that removes any water. This yields biodiesel.
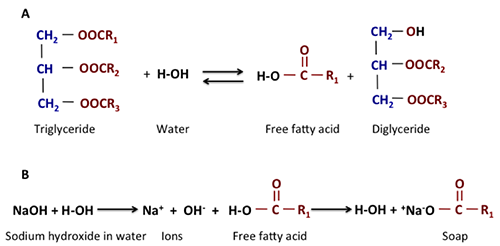
As briefly discussed, the initial reactants used in the process should be as dry as possible. Water can react with the triglyceride to make free fatty acids and a diglyceride. It can also dissociate the sodium or potassium from the hydroxide, and the ions Na+ and K+ can react with the free fatty acid to form soap. The figure below shows how water can help to form a free fatty acid, and that free fatty acid can react with the Na+ ion to form soap. The sodium that was being used for a catalyst is now bound with the fatty acid and unusable. It also complicates separation and recovery. All oils may naturally contain free fatty acids. The refined vegetable oil contains less than 1%, while crude vegetable oil has 3%, waste oil has 5%, and animal fat has 20%. Animal fats are a less desirable feedstock.