Modified Claus Process
Figure 10.4 shows the configuration of the multi-step Modified Claus Process that includes two kinds of reactors: a burner reactor and a converter reactor. In the burner reactor, H2S is burned with compressed air to SO2 and H2O. Two critically important variables of the burner reactor are the oxygen to H2S ratio and the reactor temperature. The O2/H2S ratio needs to be one-third of the stoichiometric ratio for complete combustion of H2S. The significance of the O2/H2S will be discussed further in the next section. The temperature in the burner reactor must be maintained typically at 1850°F to make sure that any ammonia present in the feed gas is completely destroyed to protect the catalysts in the converter reactor. The effluent gas from the burner reactor is cooled to 450°F (above the dew point of S) in the waste heat boiler as it enters the converter reactor for catalytic conversion of H2S and SO2 to elemental sulfur and water. The converter effluent is introduced into a condenser unit to obtain elemental sulfur as a liquid product. Small quantities of S produced in the burner reactor may also be recovered after the waste heat boiler. Typically, three sets of converter-condenser units in series are needed to achieve 95% recovery of S in the Modified Claus Process.

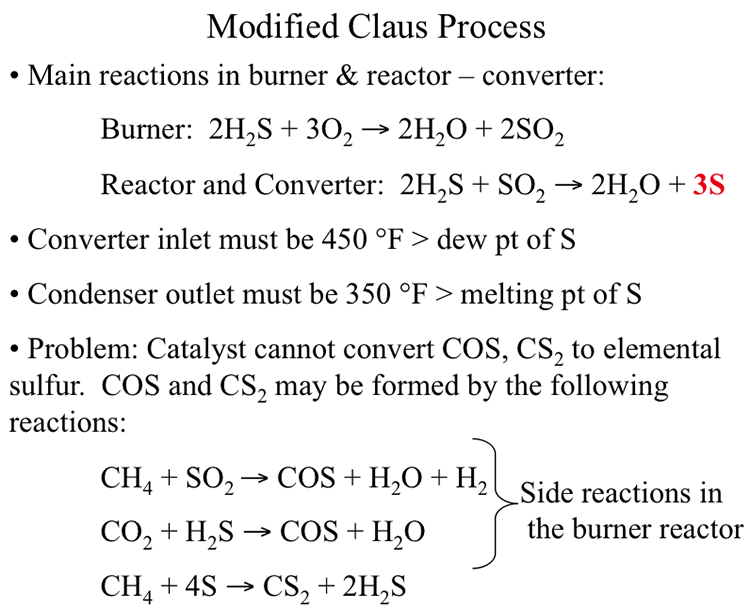
Figure 10.5 shows the principal reactions in the Modified Claus Process in the burner and converter-reactor sections. In the burner, H2S is partially oxidized to produce H2O and SO2. In the reactor converter, the burner product SO2 reacts with the remaining H2S to produce elemental sulfur (the intended product in the sulfur recovery process) along with the side product water. Ideally, the final products should consist only of elemental sulfur and water with no H2S or SO2 present. The only way to achieve the intended product mix is to control the O2/H2S ratio in the burner. As can be seen, the stoichiometric ratio of O2/H2S for complete conversion of H2S to SO2 is 3/2 which would effectively convert all H2S to SO2. In order to reserve part of the feed H2S to react with the burner product so that no H2S or SO2 remains in the final product from the converter, the O2/H2S ratio should be controlled at 1/3 of the stoichiometric ratio, that is (1/3)(3/2)=1/2. As a self-check exercise, explain with chemical equations why the desired oxygen/hydrogen sulfide ratio in the feed to the Burner in the Claus Process should be 1/3 of the stoichiometric oxygen/hydrogen sulfide ratio (for complete combustion of hydrogen sulfide). The answer to this exercise is given at the end of the lesson.
As seen in Figure 10.5, the side reactions in the burner produce COS and CS2 which cannot be converted in the catalytic reactions that take place in the converter reactor. Therefore, a tail gas clean-up process, or SCOT Process, is needed to reduce the concentration of these side products to less than 20 ppm by volume in the outlet.
Modified Claus Process
Main reactions in burner & reactor – converter:
Burner: 2H2S + 3O2 → 2H20 + 2SO2
Reactor and Converter 2H2S + SO2 → 2H2O + 3S
Converter inlet must be 450ºF > dew point of S
Condenser outlet must be 350ºF > melting point of S
Problem: Catalyst cannot convert COS, CS2 to elemental sulfur. COS and CS2 may be formed by the following reactions
CH4 + SO2 → COS + H20 + H2 (Side reaction in the burner reactor)
CO2 + H2S → COS + H20 (Side reaction in the burner reactor)
CH4 + 4S → CS2 + 2H2S