Polymerization
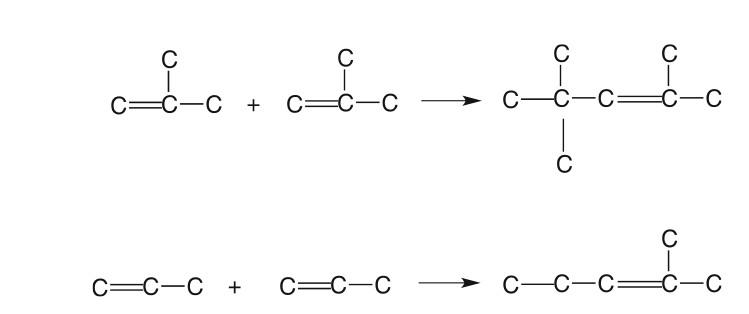
The polymerization process combines propenes and butenes to produce higher olefins with high-octane numbers (97 RON and 83 MON) for the gasoline pool. The polymerization process was used extensively in the 1930s and 1940s, but it was replaced to a large extent by the alkylation process after World War II. It has gained favor after phasing out the addition of tetraethyl lead (TEL) to gasoline, and the demand for unleaded gasoline has increased. Typical polymerization reactions are shown in Figure 8.9 [5].
The most commonly licensed polymerization process is the UOP polymerization process, which uses phosphoric acid as catalyst. IFP licenses a Dimersol® process that produces dimers from propene or butene using a homogeneous aluminum alkyl catalyst.