There are four basic polymer structures which are shown in the figure below. In practice, some polymers might contain a mixture of the various basic structures. The four basic polymer structures are linear, branched, crosslinked, and networked.
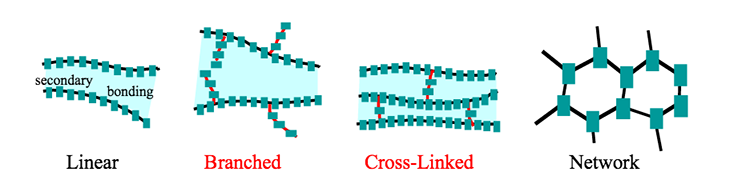
Linear polymers resemble ‘spaghetti’ with long chains. The long chains are typically held together by the weaker van der Waals or hydrogen bonding. Since these bonding types are relatively easy to break with heat, linear polymers are typically thermoplastic. Heat breaks the bonds between the long chains allowing the chains to flow past each other, allowing the material to be remolded. Upon cooling the bonds between the long chains reform, i.e., the polymer hardens.
Branched polymers resemble linear polymers with the addition of shorter chains hanging from the spaghetti backbone. Since these shorter chains can interfere with efficient packing of the polymers, branched polymers tend to be less dense than similar linear polymers. Since the short chains do not bridge from one longer backbone to another, heat will typically break the bonds between the branched polymer chains and allow the polymer to be a thermoplastic, although there are some very complex branched polymers that resist this ‘melting’ and thus break up (becoming hard in the process) before softening, i.e., they are thermosetting.
Crosslinked polymers resemble ladders. The chains link from one backbone to another. So, unlike linear polymers which are held together by weaker van der Waals forces, crosslinked polymers are tied together via covalent bonding. This much stronger bond makes most crosslinked polymers thermosetting, with only a few exceptions to the rule: crosslinked polymers that happen to break their crosslinks at relatively low temperatures.
Networked polymers are complex polymers that are heavily linked to form a complex network of three-dimensional linkages. These polymers are nearly impossible to soften when heating without degrading the underlying polymer structure and are thus thermosetting polymers.
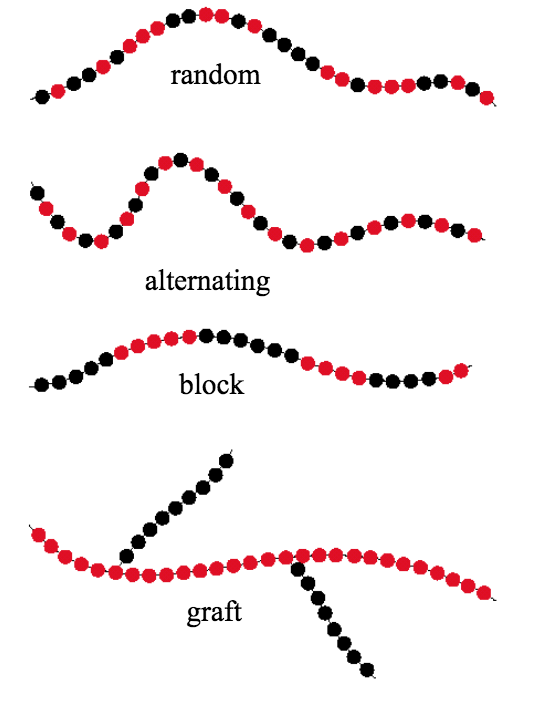
Monomers do not have to be of a single atom type, but when referring to a specific monomer it is understood to be of the same composition structure. When building a polymer from two distinct monomers, those polymers are referred to as copolymers. Next, we will look at how copolymers are classified.
Copolymers
If a chemist is synthesizing a polymer utilizing two distinct starting monomers there are several possible structures, as shown in the figure below. The four basic structures are random, alternating, block, and graft. If the two monomers are randomly ordered then the copolymer is, not surprisingly, referred to as a random copolymer. In an alternating copolymer, each monomer is alternated with the other to form an ABABABA… pattern. In block copolymers, more complex repeating structures are possible, for example AAABBBAAABBBAAA… Graft copolymers are created by attaching chains of a second type of monomer on the backbone chain of a first monomer type.
Before we move on to the many uses of polymers, watch this four-minute video which will introduce the uses of polymers.
To Watch
In our previous videos we have explored how polymers are formed and equations for polymerization reactions. In this video, we'll explore in more detail some different polymers and their specific uses as well as the problems associated with polymers. As you now know, polymers are a long chain of organic molecules made by repeating monetary units. There are a number of natural polymers in life such as rubber, and even in our own body we have natural polymers such as proteins, carbohydrates, and DNA to name just a few. We'll focus the rest of this tutorial on synthetic polymers. The common name to synthetically made polymers is plastics which are used very frequently in our day-to-day lives. From simple packaging to complex structural building materials. However, the increased use of plastics in our homes leads to nearly one-quarter of all the solid waste being plastic. Some of this can be recycled to minimize the effects on our environment. It's a long-term goal for many chemists is to develop more biodegradable plastics which would naturally break down in our environment. Here are some specific examples of polymers and their common uses.
Polythene used for carrier bags and plastics. High-density polyethylene is used for drain pipes, water bottles, and containers. Polystyrene is used in packaging. Polypropylene is used for bottle caps, plastic bottles, and plastic pipes. Polychloroprene Etaene, often known as PVC, is used for windows and door frames, plastic hinges, and bottles. Polly 1122 tetrafluoroethylene also known as PTFE, which is a nonstick coating on frying pans as well as being used in bearings another low friction surfaces. Kevlar is a unique polymer in that it is used for bulletproof vests and jackets. Nylon is used in textiles, clothing, and carpets. As you can see polymers play a huge role in our day-to-day lives and their use is wide and varied owing to their unique individual properties. It is important to understand the most of the alkene monomers used to make polymers are obtained in some part from crude oil and therefore, it is critical that we recycle plastics to conserve our natural resources for the future manufacture of these polymers.
There are also big problems associated with the disposal of polymers. The biggest problem as mentioned above is that polymers are non-biodegradable which means that microorganisms cannot naturally break them down. Disposal of polymers by burning or incineration is a possibility, as this generates heat which can be used to generate electricity. However, the burning of polymers produces many toxic gasses which themselves can damage the environment and cause pollution.
Now at the end of this lesson, you should have an appreciation of the importance of polymers, be able to name some key polymers along with their uses, and also describe the problems associated with polymers.
Now that you have watched this video, please proceed to the second (of two) reading assignments for this lesson.