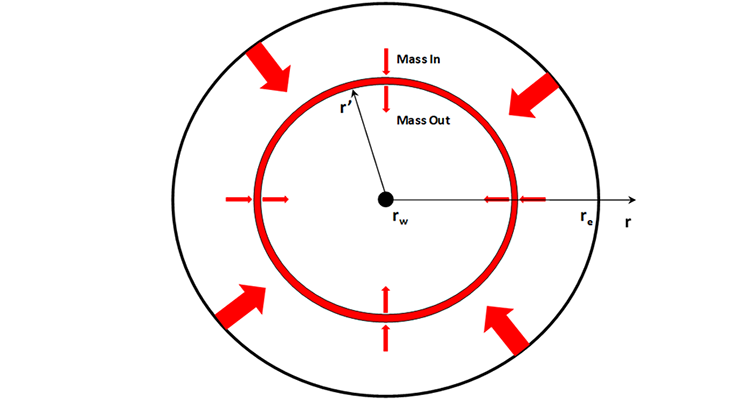
As with the flow of oil, we begin the derivation of diffusivity equation for compressible gas flow with a mass balance on a thin ring or Representative Elemental Volume, REV, in the reservoir as shown in Figure 5.05. This mass balance results in:
We can elaborate on the definitions of terms in Equation 5.26 as:
and,
Where:
- is the gas density, lb/ft3
- is the gas rate, ft3/day
- is the radial coordinate in a radial-cylindrical coordinate system, ft
- is the radius of the representative elemental volume, REV, ft
- is time, days
- is the porosity of the reservoir, fraction
- is the bulk volume of the representative elemental volume, REV, ft3
- is the reservoir thickness, ft
Substituting Equation 5.27 through Equation 5.29 into Equation 5.26 results in:
or,
Dividing by the term results in:
Now, substituting Darcy’s Law, Equation 5.06 with and without the formation volume factor, , (we want the flow rate in reservoir ft3/day not SCF/day). The unit conversion factor of 5.615 ft3/bbl converts Darcy’s Law from bbl/day to ft3/day:
If we assume that the permeability, , and the thickness, , are uniform, then we have:
or,
To this point, the derivation for the compressible gas equation is identical to the derivation for a slightly compressible liquid equation. This derivation will begin to deviate now. Applying the definition of density for a real gas (Equation 3.71):
Equation 5.34 is a direct result of the Real Gas Law. Substituting into Equation 5.33b results in:
or,
or,
Now, we saw in Lesson 3 that for a real gas, the definition of compressibility is (Equation 3.70b):
or,
Substituting into Equation 5.35c results in:
Equation 5.37 is the nonlinear diffusivity equation describing the transient behavior of compressible (real) gases. We say that it is Nonlinear because of the functions of pressure appearing in the equation. In this nonlinear form, we cannot solve the equation analytically (exactly). In order to obtain analytical solutions to this equation, we must first Linearize it. We do this in the same manner as we linearized the stabilized flow equations: by use of the Pressure, the Pressure-Squared, and the Pseudo-Pressure Formulations.