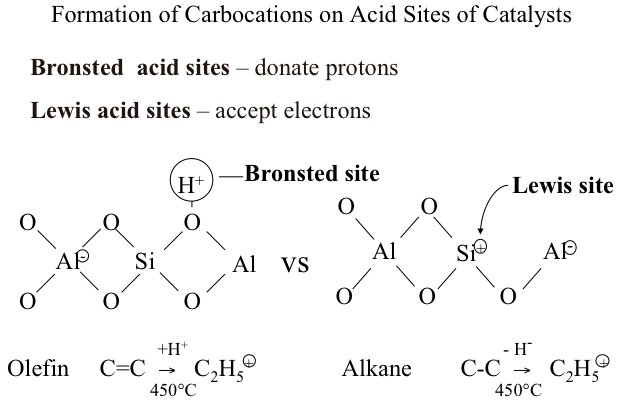
Carbocations are formed from hydrocarbons on two different acid sites: Bronsted acid sites and Lewis acid sites. You should remember that Bronsted acid sites donate protons, while Lewis acid sites accept electrons to form carbocations from hydrocarbons. Figure 7.2 illustrates how an olefin (e.g., ethylene, C=C) produces an ethyl carbenium ion (C+2H5) by reacting with a proton donated from Bronsted acid site. Alternatively, also seen in Figure 7.2, a Lewis acid site accepts an electron (or a hydride ion, H-) from an alkane (e.g., ethane, C-C) to produce the same ethyl carbenium ion (C+2H5). These two reactions that take place on the acid sites of catalysts, along with the formation of carbonium ions by protonation of hydrocarbons on Bronsted sites, function as the initiation steps in the ionic chain reactions that lead to the products obtained from catalytic cracking.