Chemistry of Catalytic Cracking
As opposed to thermal cracking governed by free radicals, catalytic cracking proceeds through the formation of ionic species on catalyst surfaces, and produces shorter, but branched-chain (not straight-chain) alkanes by cracking the long straight-chain alkanes. The formation of branched-chain alkanes, or iso-alkanes, leads to the production of gasoline with high octane numbers. This is the fundamental reason why catalytic cracking has replaced thermal cracking as the central process in a refinery geared to maximize gasoline production. A high octane number of gasoline is needed for current spark-ignition engines to run at high compression ratios without knocking. High compression ratios in spark-ignition engines translate to high power and high efficiency.
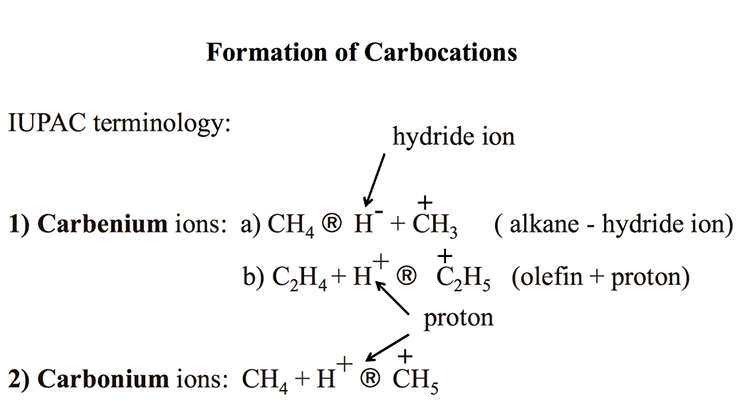
Figure 7.1 introduces the two types of ionic species, carbocations, that are active in catalytic cracking reactions as carbenium, and carbonium ions, using the IUPAC terminology. Carbocations are the positively charged ions made from hydrocarbons. Figure 7.1 shows that removing a hydride ion (H-, a hydrogen atom with an additional electron) from an alkane (e.g., methane) produces carbenium ions (path 1a). Also, adding a proton (H+, a hydrogen atom without the electron) to an olefin (e.g., ethylene) can produce carbenium ions, as shown in path 1b.
Formation of Carbocations
IUPAC terminology
-Carbenium ions: CH4 (arrow) H- + CH3+ (alkane – hydride ion)
-C2H4 + H+ (arrow) C2H5+. (olefin + proton)
-Carbonium Ions: CH4 + H+ (arrow) CH4
KEY: H- (hydride ion), H+ (proton)
Analogous to the terminology used for free radicals, C+H3 is called methyl carbenium ion, and C2+H5 is called an ethyl carbenium ion. Carbonium ions are produced by adding a proton to an alkane, say methane, as shown in Figure 7.1. The resulting ion C+H5 is called methanium. Note that there is some confusion in the literature about naming the carbocations. Carbenium ions used to be called carbonium ions in some sources, including your textbook [2]. All references to carbonium ions in Section 6.3 Cracking Reactions in the textbook should be corrected as carbenium ions.