Distribution of Products
Figure 7.3 compares the distribution of products from thermal cracking (free radical chain reactions) and catalytic cracking (ionic chain reactions). Short chain paraffins constitute the principal products in both cases, with one important difference – an abundance of iso-alkanes (branched-chain alkanes) in catalytic cracking products. One can also note in Figure 7.3 that catalytic cracking products contain higher concentration of aromatic compounds. High octane number of gasoline produced by catalytic cracking can be attributed to high concentrations of i-alkanes and relatively more abundant aromatics present in the crackate (catalytic cracking product). Having no olefins larger than butylene (C4) from catalytic cracking processes, also distinguishes catalytic cracking products from thermal cracking products obtained from gas oil.
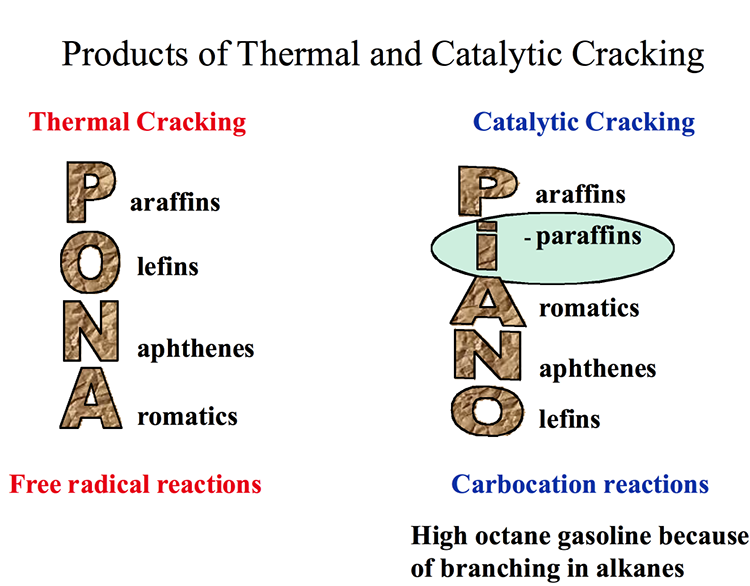
Products of Thermal and Catalytic Cracking
Thermal Cracking – Free radical reactions
P araffins
O lefins
N aphthenes
A romatics
Catalytic Cracking – Carbocation reaction
High octane gasoline because of branching in alkanes
P araffins
I -paraffins
A romatics
N aphthenes
O lefins