Ionic Chain Reactions
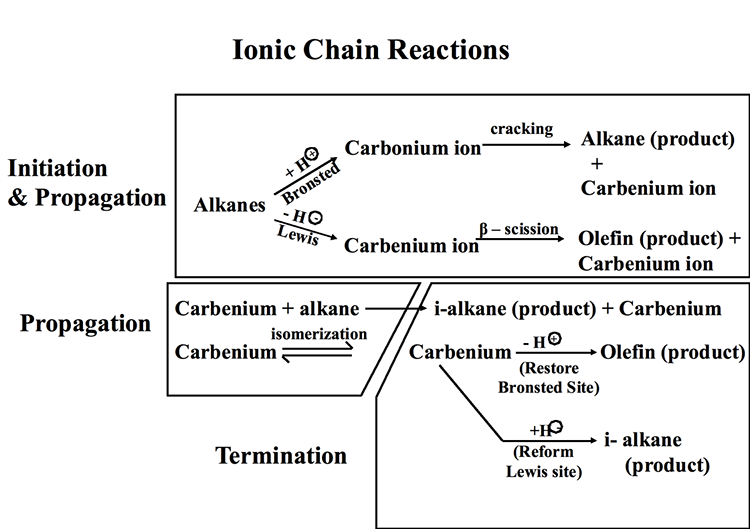
Figure 7.4 illustrates the ionic chain reactions that govern catalytic cracking of hydrocarbons. The initiation step includes the formation of a carbonium ion by proton donation from a Bronsted acid site and/or the formation of a carbenium ion through hydride ion abstraction by a Lewis acid site. In a propagation step, the carbonium ion goes through cracking to produce an alkane product and a carbenium ion, while the carbenium ion produced on the Lewis acid site goes through a β-scission to produce an olefin product and another carbenium ion. In additional propagation reactions, carbenium ions (secondary, or tertiary) react with alkanes to produce i-alkane products and other carbenium ions, which can go through isomerization reactions generating more stable ions. Finally, in termination steps, carbenium ions donate a proton to restore a Bronsted acid site and produce an olefin as final product, or they abstract a hydride ion to restore a Lewis acid site producing an i-alkane product, and the ionic chain reaction continues. Other reactions during catalytic cracking include dehydrocyclization and dehydrogenation reactions to produce aromatic compounds. One should note that thermal cracking reactions also take place during catalytic cracking because of the sufficiently high temperatures used in the process. Some claim that initial thermal cracking of alkanes to produce olefins should also be considered as an initiation step in ionic chain reactions [2].