The idea here is to determine the mass of CO2 you released into the atmosphere during the first part of your experiment. Watch your unit conversions!
First, a few assumptions:
- We shall assume that the physical properties of Diet Coke are the same as those of water (H2O) at room temperature (20˚C)
- We shall assume that a typical soda contains ~0.5% CO2 by weight prior to being opened (in reality this may vary by quite a bit)
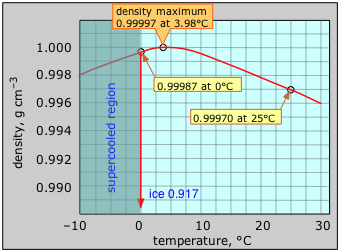
We start by determining the total mass of CO2 present at the beginning of the experiment (prior to opening the bottle). In order to do this, first you will need to determine the mass of Diet Coke. Use the graph below to determine the density of water at 20˚C; we will assume your Diet Coke has the same density. Note that 1 cm3 = 1 mL.
- Approximately how many grams of Diet Coke (water) are in a 2 liter bottle?
- Given that the concentration of CO2 in soda is ~0.5% by weight, how many grams of CO2 are in the bottle?
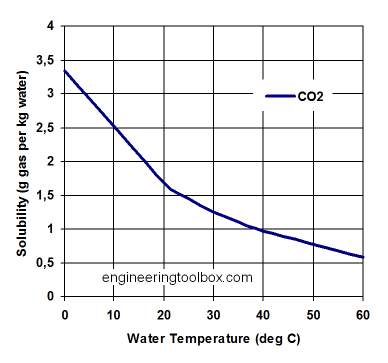
Solubility is the amount of a compound that will remain in solution under a given set of conditions. Use the graph below to estimate the solubility of CO2 in water at 20˚C and atmospheric pressure.
The amount of CO2 released is given by the total amount present prior to opening the bottle minus the amount retained after the degassing experiment.
- How many grams of CO2 will remain dissolved in our 2 liters of Diet Coke after the experiment is done?
- What was the mass of CO2 released?