Batteries are the final commercial product that are delivered to customers and that require some data provided from the manufacturers to allow customers to evaluate the performance of different battery types in terms of capacity rating, allowable DOD, and temperature operating ranges. Most datasheets come with some curves that a PV designer should be able to perfectly interpret for best design practices.
In this section, we will discuss basic parameters of batteries and main factors that affect the performance of the battery.
The first important parameters are the voltage and capacity ratings of the battery.
Every battery comes with a certain voltage and capacity rating. As briefly discussed earlier, there are cells inside each battery that form the voltage level, and that battery rated voltage is the nominal voltage at which the battery is supposed to operate.
The capacity refers to the amount of charge that the battery can deliver at the rated voltage, which is directly proportional to the amount of electrode material in the battery.
The unit for measuring battery capacity is ampere-hour or amp-hour, denoted as (Ah). The capacity can also be expressed in terms of energy capacity of the battery. The energy capacity is the rated battery voltage in volts multiplied by battery capacity in amp-hours, giving total battery energy capacity in watt-hours (wh). In general, it is the total amount of energy that the device can store.
You must be wondering what is the significance of amp-hours as the unit of battery capacity? The unit itself gives us some important clues about battery properties. A brand new battery with a 100 amp-hour capacity can theoretically deliver a 1 A current for 100 hours at room temperature. In practice, this is not the case due to several factors, as we will see later.
C-rate
Let's move to another important battery parameter, called the C-rate. C-rate is the discharge rate of the battery relative to its capacity. The C-rate "number" is nothing but the discharge current, at which the battery is being discharged, over the nominal battery capacity. It is calculated as the following:
Where
"Idis" is the discharge current
"Cnon" is the nominal battery capacity
The discharge rate is sometimes referred to as C/”number” and that number is the number of hours it takes the battery to be fully discharged. In other words, it is the inverse of the previous notation, and it is calculated as the following:
For example, a C-rate of 1C for 100 Ah capacity battery would correspond to a discharge current of 100 A over 1 hour. Or it can be represented as C/1. On the other hand, a C-rate of 2C for the same battery would correspond to a discharge current of 200 A over half an hour. Or it can be represented as C/0.5. Similarly, a C-rate of 0.05C implies a discharge current of 5 A over 20 hours. Or it can be represented as C/20. Finally, the same battery can be discharged at 1 A over 100 hours, and that corresponds to 0.01C or C/100. In general, C-rate depends on charging and discharging current.
Efficiency
Since there is no energy conversion system that is 100% efficient, the term efficiency represents the system capability to transfer energy from the input of the system to the output. Each battery type comes with different efficiency rating as discussed in EME 812 (9.3. Battery storage - Table 9.1), and usually we talk about efficiencies of both charge and discharge combined.
Battery efficiency is the ratio of total storage system input to the total storage system output. For example, if 10 kWh is pumped into the battery while charging, and you can effectively retrieve only 8 kWh while discharging, then the round trip efficiency of the storage system is 80%.
Let's discuss another important battery parameter, the state of charge or SOC. It is defined as the percentage of the battery capacity available for discharge, so thus, a 100 Ah rated battery that has been drained by 20 Ah had an SOC of 80%. Another parameter that complements the SOC is the depth of discharge or DOD, which is the percentage of the battery capacity that has been discharged. Thus, a 100 Ah battery that has been drained by 20 Ah has a DOD of 20%. In other words, the DOD and SOC are complementary to one another.
Now we come to a very important parameter: the cycle lifetime of the battery. Cycle lifetime is defined as the number of charging and discharging cycles after which the battery capacity drops below 80% of the nominal value. Usually, the cycle life is specified as an absolute number. However, to be more precise, cycle life and other battery parameters are affected by changing ambient condition such (temperature in this case).
So what is the relationship between the battery parameters? The cycle life depends heavily on the depth of discharge. This can be seen in Figure 3.6 for a typical flooded lead-acid battery. If we look at the effective capacity at different depth of discahrge (DOD) rates for a lead-acid battery, we can see that the cycle number diminishes as the DOD increases.
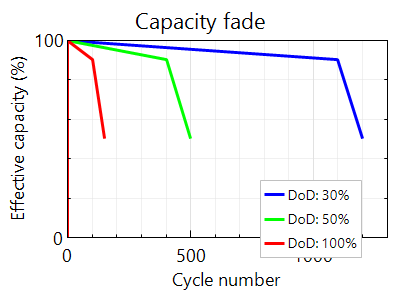
Cycle lifetime also depends on the temperature. The battery lasts longer under colder temperatures of operation. Furthermore, we can observe from Figure 3.6 that for a particular temperature, cycle lifetime depends non-linearly on the depth of discharge. The smaller the DOD, the higher the cycle lifetime. However, such a higher cycle life would also mean that those additional cycles you gain can only help you for a smaller depth of discharge. Thus, it could be said that the battery will last longer if the average DOD could be reduced over its normal operation. Also, battery overheating should be strictly controlled. Overheating could occur due to overcharging and subsequent overvoltage of the lead-acid battery. We will learn more about voltage and charge control of the battery in the next section.
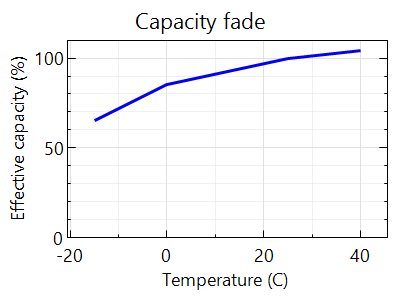
While battery life is increased at lower temperatures, there is one more effect that needs to be considered. The temperature affects battery capacity during regular use, too. As seen in Figure 3.7, the lower the temperature, the lower the battery capacity. The Higher the temperature, the higher the battery capacity.
Reflection
Why does the capacity increase with temperature?
ANSWER: This is because, at high temperatures, the chemicals in the battery are more active, and therefore chemical activity tends to increase the battery capacity. Contrarily, the chemical activity is hampered at lower temperatures that lower the capacity
Battery Aging Factors
It might not seem scientific, but it is even possible to reach an above rated capacity of the battery at high temperatures. However, such high temperatures are severely detrimental to battery health.
When we say that a battery has a limited cycle life, or that it has completely "run out of juice," what exactly does that mean? Is it related to the aging effect of the lead-acid battery?
There are several factors that contribute to the aging of any battery. Sulphation is one of the major causes of aging. And if the battery is not fully recharged after being heavily discharged, that causes sulphate crystals to grow, which cannot be completely transformed back into lead or lead oxide. As a result, the battery slowly loses the mass of active material and therefore discharge capacity will be lower. Corrosion of lead grid at the electrode is another common aging factor. This leads to increased grid resistance due to high positive potentials.
Moving further, when the battery loses moisture, it causes the electrolyte to dry out, which occurs at high charging voltages, resulting in loss of water. It is referred to as gassing effect and may limit battery lifetime. This should be taken care of with routine maintenance by adding distilled water to the battery.
Researchers have developed maintenance free lead-acid batteries for solar systems that exhibit very high lifetimes. However, these are also high-end products and can be more expensive.
Reflection
How do we determine if the battery is preferred to be a maintenance-free type when designed for PV applications?
ANSWER: It is decided by the number of batteries required and the accessibility for routine maintenance. It is preferred to have maintenance-free batteries when the installation is not easily accessible or if the system requires a large number of batteries.
After we covered all basic battery parameters and characteristic curves, a designer should be able to make the best selection for a product depending on the application. But how do designers put these batteries in place? Is there only one size for all batteries, and it is scalable? Or do they make a custom design battery for each project? We will answer these questions in the next section.