6.3a Composition of Corn and Yield of Ethanol from Corn
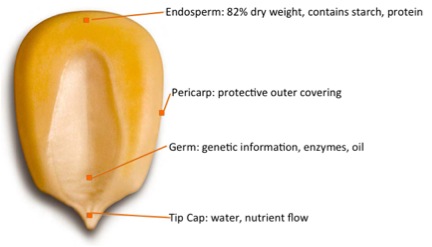
As established in the previous section, corn has the least expensive total cost for ethanol production. So what part of the corn is used for ethanol? Primarily the corn kernel is used for ethanol production. The figure below shows the general composition of corn. It is a picture of yellow dent corn, which is commonly used for ethanol production. The endosperm is mostly composed of starch, the corn’s energy storage, and protein for germination. It is the starch that is used for making fuel. The pericarp is the outer covering that protects the kernel and preserves the nutrients inside. The pericarp resists water and water vapor and protects against insects and microorganisms. The living organism in the kernel is the germ. It contains genetic information, enzymes, vitamins, and minerals, which help the kernels grow into a corn plant. About 25% of the germ is corn oil and is a valuable part of the kernel. The tip cap is where the kernel is attached to the cob, and water and nutrients flow through the tip cap. This part of the kernel is not covered by the pericarp.
Starch is a polymer. It is made up of D-glucose units. Therefore, glucose components directly impact ethanol yields. The components of yellow dent corn are the following. It is primarily composed of starch, at 62%. The corn kernel is also composed of protein and fiber (19%), water (15%), and oil (4%). It can also contain traces of other constituents, but these are small relative to the main components. If you’ll recall from Lesson 6, starch is composed of two different polymeric molecules: amylose and amylopectin. If you factor in these two carbons, the starch can be broken into these components: amylopectin is 50% of the yellow dent corn kernel (80% of the starch) and amylose is 12% of the kernel (20% of the starch).
One bushel of corn (56 lbs.) can provide several products. The one bushel can provide:
31.5 lbs. of starch
OR
33 lbs. of sweetener
OR
2.8 gal. of fuel ethanol
OR
22.4 lbs of PLA fiber, which is a starch-based polymer called polylactic acid
In addition, the corn will provide 13.5 lbs. of gluten feed (20% protein), 2.5 lbs. of gluten meal (60% protein), and 1.5 lbs. of corn oil. Based on this information, we can calculate the actual yield to the theoretical yield and determine the percent yield we can achieve for ethanol conversion. This is shown below:
1 bushel of corn:
56 lbs/bu x 62% starch = 34.7 lbs of starch/bu
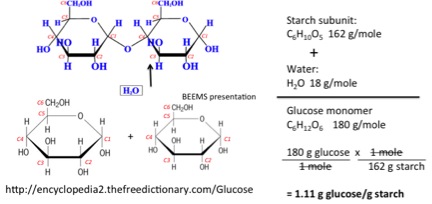
34.7 lbs starch x 1.11 lbs glucose/lb starch = 38.5 lbs glucose/bu
The reaction of glucose to ethanol:
38.5 lbs glucose x 92 lbs EtOH/180 lbs glucose = 19.7 lbs EtOH/bu
19.7 lbs EtOH x 1 gal EtOH/6.6 lbs = 3.0 gal EtOH/bu theoretical
100 x 2.8/3.0 = 93% yield of ethanol, typically
As discussed in Lesson 5 for pretreatment of lignocellulosic biomass, the breaking down of glucose also requires hydrolysis. As water ionizes into H+ and OH-, it will break apart a molecule such as maltose into two glucose molecules. The reaction does not happen fast without either an enzyme (Lesson 6) or acid/heat (Lesson 5). The figure below shows the ratio of glucose monomer to the glucose subunit in starch. When starch is broken down, it is done by adding the water molecule to form the glucose. This is where the value for lbs glucose/lb starch is derived for the calculation above.